BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways
- PMID: 19793887
- PMCID: PMC2761108
- DOI: 10.1242/dev.038133
BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways
Abstract
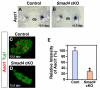
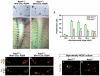
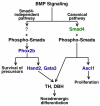
Induction of the sympathetic nervous system (SNS) from its neural crest (NC) precursors is dependent on BMP signaling from the dorsal aorta. To determine the roles of BMP signaling and the pathways involved in SNS development, we conditionally knocked out components of the BMP pathways. To determine if BMP signaling is a cell-autonomous requirement of SNS development, the Alk3 (BMP receptor IA) was deleted in the NC lineage. The loss of Alk3 does not prevent NC cell migration, but the cells die immediately after reaching the dorsal aorta. The paired homeodomain factor Phox2b, known to be essential for survival of SNS precursors, is downregulated, suggesting that Phox2b is a target of BMP signaling. To determine if Alk3 signals through the canonical BMP pathway, Smad4 was deleted in the NC lineage. Loss of Smad4 does not affect neurogenesis and ganglia formation; however, proliferation and noradrenergic differentiation are reduced. Analysis of transcription factors regulating SNS development shows that the basic helix-loop-helix factor Ascl1 is downregulated by loss of Smad4 and that Ascl1 regulates SNS proliferation but not noradrenergic differentiation. To determine if the BMP-activated Tak1 (Map3k7) pathway plays a role in SNS development, Tak1 was deleted in the NC lineage. We show that Tak1 is not involved in SNS development. Taken together, our results suggest multiple roles for BMP signaling during SNS development. The Smad4-independent pathway acts through the activation of Phox2b to regulate survival of SNS precursors, whereas the Smad4-dependent pathway controls noradrenergic differentiation and regulates proliferation by maintaining Ascl1 expression.
Figures





References
-
- Chalazonitis, A., D'Autreaux, F., Guha, U., Pham, T. D., Faure, C., Chen, J. J., Roman, D., Kan, L., Rothman, T. P., Kessler, J. A. et al. (2004). Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J. Neurosci. 24, 4266-4282. - PMC - PubMed
-
- D'Autreaux, F., Morikawa, Y., Cserjesi, P. and Gershon, M. D. (2007). Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development 134, 2237-2249. - PubMed
-
- Dai, Y. S., Hao, J., Bonin, C., Morikawa, Y. and Cserjesi, P. (2004). JAB1 enhances HAND2 transcriptional activity by regulating HAND2 DNA binding. J. Neurosci. Res. 76, 613-622. - PubMed
-
- Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K. and McMahon, A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

