Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions
- PMID: 19793892
- PMCID: PMC2761109
- DOI: 10.1242/dev.035857
Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions
Abstract
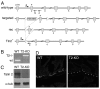
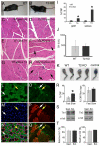
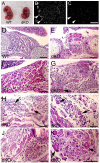
Talin 1 and 2 connect integrins to the actin cytoskeleton and regulate the affinity of integrins for ligands. In skeletal muscle, talin 1 regulates the stability of myotendinous junctions (MTJs), but the function of talin 2 in skeletal muscle is not known. Here we show that MTJ integrity is affected in talin 2-deficient mice. Concomitant ablation of talin 1 and 2 leads to defects in myoblast fusion and sarcomere assembly, resembling defects in muscle lacking beta1 integrins. Talin 1/2-deficient myoblasts express functionally active beta1 integrins, suggesting that defects in muscle development are not primarily caused by defects in ligand binding, but rather by disruptions of the interaction of integrins with the cytoskeleton. Consistent with this finding, assembly of integrin adhesion complexes is perturbed in the remaining muscle fibers of talin 1/2-deficient mice. We conclude that talin 1 and 2 are crucial for skeletal muscle development, where they regulate myoblast fusion, sarcomere assembly and the maintenance of MTJs.
Figures




Similar articles
-
Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle.Development. 2008 Jun;135(11):2043-53. doi: 10.1242/dev.015818. Epub 2008 Apr 23. Development. 2008. PMID: 18434420 Free PMC article.
-
Beta1 integrins regulate myoblast fusion and sarcomere assembly.Dev Cell. 2003 May;4(5):673-85. doi: 10.1016/s1534-5807(03)00118-7. Dev Cell. 2003. PMID: 12737803
-
Assembly of myotendinous junctions in the chick embryo: deposition of P68 is an early event in myotendinous junction formation.Dev Biol. 1994 Jun;163(2):447-56. doi: 10.1006/dbio.1994.1161. Dev Biol. 1994. PMID: 8200480
-
Talin - the master of integrin adhesions.J Cell Sci. 2017 Aug 1;130(15):2435-2446. doi: 10.1242/jcs.190991. Epub 2017 Jul 12. J Cell Sci. 2017. PMID: 28701514 Review.
-
Force-Directed "Mechanointeractome" of Talin-Integrin.Biochemistry. 2019 Nov 26;58(47):4677-4695. doi: 10.1021/acs.biochem.9b00442. Epub 2019 Aug 21. Biochemistry. 2019. PMID: 31393109 Review.
Cited by
-
Integrin inactivators: balancing cellular functions in vitro and in vivo.Nat Rev Mol Cell Biol. 2013 Jul;14(7):430-42. doi: 10.1038/nrm3599. Epub 2013 May 30. Nat Rev Mol Cell Biol. 2013. PMID: 23719537 Review.
-
The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices.Biomaterials. 2012 Oct;33(29):6943-51. doi: 10.1016/j.biomaterials.2012.06.057. Epub 2012 Jul 15. Biomaterials. 2012. PMID: 22800539 Free PMC article.
-
Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics.Hum Mol Genet. 2014 Sep 15;23(18):4745-57. doi: 10.1093/hmg/ddu189. Epub 2014 Apr 23. Hum Mol Genet. 2014. PMID: 24760765 Free PMC article.
-
Are mechanically sensitive regulators involved in the function and (patho)physiology of cerebral palsy-related contractures?J Muscle Res Cell Motil. 2017 Aug;38(3-4):317-330. doi: 10.1007/s10974-017-9489-1. Epub 2017 Nov 30. J Muscle Res Cell Motil. 2017. PMID: 29190010 Review.
-
Talin1 regulates integrin turnover to promote embryonic epithelial morphogenesis.Mol Cell Biol. 2011 Aug;31(16):3366-77. doi: 10.1128/MCB.01403-10. Epub 2011 Jun 13. Mol Cell Biol. 2011. PMID: 21670148 Free PMC article.
References
-
- Araya, R., Eckardt, D., Maxeiner, S., Kruger, O., Theis, M., Willecke, K. and Saez, J. C. (2005). Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J. Cell Sci. 118, 27-37. - PubMed
-
- Balaban, N. Q., Schwarz, U. S., Riveline, D., Goichberg, P., Tzur, G., Sabanay, I., Mahalu, D., Safran, S., Bershadsky, A., Addadi, L. et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466-472. - PubMed
-
- Bazzoni, G., Shih, D. T., Buck, C. A. and Hemler, M. E. (1995). Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570-25577. - PubMed
-
- Belkin, A. M., Zhidkova, N. I., Balzac, F., Altruda, F., Tomatis, D., Maier, A., Tarone, G., Koteliansky, V. E. and Burridge, K. (1996). Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J. Cell Biol. 132, 211-226. - PMC - PubMed
-
- Belkin, A. M., Retta, S. F., Pletjushkina, O. Y., Balzac, F., Silengo, L., Fassler, R., Koteliansky, V. E., Burridge, K. and Tarone, G. (1997). Muscle beta1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J. Cell Biol. 139, 1583-1595. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

