The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites
- PMID: 19793917
- PMCID: PMC2777107
- DOI: 10.1091/mbc.e09-07-0643
The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites
Abstract
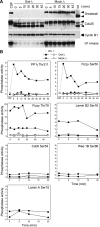
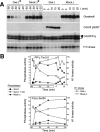
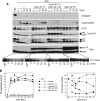
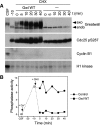
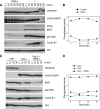
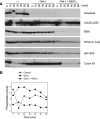
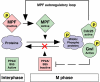
We have previously shown that Greatwall kinase (Gwl) is required for M phase entry and maintenance in Xenopus egg extracts. Here, we demonstrate that Gwl plays a crucial role in a novel biochemical pathway that inactivates, specifically during M phase, "antimitotic" phosphatases directed against phosphorylations catalyzed by cyclin-dependent kinases (CDKs). A major component of this phosphatase activity is heterotrimeric PP2A containing the B55delta regulatory subunit. Gwl is activated during M phase by Cdk1/cyclin B (MPF), but once activated, Gwl promotes PP2A/B55delta inhibition with no further requirement for MPF. In the absence of Gwl, PP2A/B55delta remains active even when MPF levels are high. The removal of PP2A/B55delta corrects the inability of Gwl-depleted extracts to enter M phase. These findings support the hypothesis that M phase requires not only high levels of MPF function, but also the suppression, through a Gwl-dependent mechanism, of phosphatase(s) that would otherwise remove MPF-driven phosphorylations.
Figures




References
-
- Agostinis P., Derua R., Sarno S., Goris J., Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP, Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by p34Cdc2 kinase. Eur. J. Biochem. 1992;205:241–248. - PubMed
-
- Che S., Wu W., Nelman-Gonzalez M., Stukenberg T., Clark R., Kuang J. A phosphatase activity in Xenopus oocyte extracts preferentially dephosphorylates the MPM-2 epitope. FEBS Lett. 1998;424:225–233. - PubMed
-
- Eto M., Karginov A., Brautigan D. L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

