Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat
- PMID: 19793959
- PMCID: PMC2803618
- DOI: 10.1152/ajpregu.00461.2009
Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat
Abstract
Understanding the early factors affecting obesity development in males and females may help to prevent obesity and may lead to the discovery of more effective treatments for those already obese. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat model of obesity is characterized by hyperphagia-induced obesity, due to a spontaneous lack of CCK(1) receptors. In the present study, we focused on the behavioral and physiological aspects of obesity development from weaning to adulthood. We examined body weight, feeding efficiency, fat pad [brown, retroperitoneal, inguinal and epydidimal (in males)] weight, inguinal adipocyte size and number, leptin and oxytocin levels, body mass index, waist circumference, and females' estrous cycle structure. In the males, central hypothalamic gene expression was also examined. OLETF rats presented overall higher fat and leptin levels, larger adipocytes, and increased waist circumference and BMI from weaning until adulthood, compared with controls. Analysis of developmental patterns of gene expression for hypothalamic neuropeptides revealed peptide-specific patterns that may underlie or be a consequence of the obesity development. Analysis of the developmental trajectories toward obesity within the OLETF strain revealed that OLETF females developed obesity in a more gradual manner than the males, presenting delayed obesity-related "turning points," with reduced adipocyte size but larger postweaning fat pads and increased adipocyte hyperplasia compared with the males. Intake decrease in estrus vs. proestrus was significantly less in OLETF vs. Long-Evans Tokushima Otsuka females. The findings highlight the importance of using different sex-appropriate approaches to increase the efficacy of therapeutic interventions in the treatment and prevention of chronic early-onset obesity.
Figures
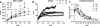



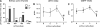
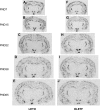
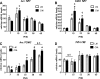
 P < 0.05 within the OLETF strain (significantly different from all other ages); #P < 0.05 for strain differences.
P < 0.05 within the OLETF strain (significantly different from all other ages); #P < 0.05 for strain differences.References
-
- Applegate EA, Upton DE, Stern JS. Food intake, body composition and blood lipids following treadmill exercise in male and female rats. Physiol Behav 28: 917– 920, 1982 - PubMed
-
- Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing—a quick, simple method. J Lipid Res 17: 190– 192, 1976 - PubMed
-
- Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther 4: 871– 895, 2006 - PubMed
-
- Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 6: 343– 368, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

