Mitochondrial Ca2+ cycling facilitates activation of the transcription factor NFAT in sensory neurons
- PMID: 19793968
- PMCID: PMC2805078
- DOI: 10.1523/JNEUROSCI.3384-09.2009
Mitochondrial Ca2+ cycling facilitates activation of the transcription factor NFAT in sensory neurons
Abstract
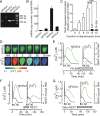
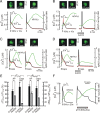
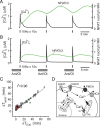
Ca(2+)-dependent gene regulation controls many aspects of neuronal plasticity. Significant progress has been made toward understanding the roles of voltage- and ligand-gated Ca(2+) channels in triggering specific transcriptional responses. In contrast, the functional importance of Ca(2+) buffers and Ca(2+) transporters in neuronal gene regulation is less clear despite their critical contribution to the spatiotemporal control of Ca(2+) signals. Here we examined the role of mitochondrial Ca(2+) uptake and release in regulating the Ca(2+)-dependent transcription factor NFAT (nuclear factor of activated T-cells), which has been implicated in synaptic plasticity, axonal growth, and neuronal survival. Intense stimulation of sensory neurons by action potentials or TRPV1 agonists induced rapid activation and nuclear import of NFAT. Nuclear translocation of NFAT was associated with a characteristic prolonged [Ca(2+)](i) elevation (plateau) that resulted from Ca(2+) uptake by, and its subsequent release from, mitochondria. Measurements using a mitochondrial Ca(2+) indicator, mtPericam, showed that this process recruited mitochondria throughout the cell body, including the perinuclear region. [Ca(2+)](i) levels attained during the plateau phase were similar to or higher than those required for NFAT activation (200-300 nm). The elimination of the [Ca(2+)](i) plateau by blocking either mitochondrial Ca(2+) uptake via the uniporter or Ca(2+) release via the mitochondrial Na(+)/Ca(2+) exchanger strongly reduced nuclear import of NFAT. Furthermore, preventing Ca(2+) mobilization via the mitochondrial Na(+)/Ca(2+) exchanger diminished NFAT-mediated transcription. Collectively, these data implicate activity-induced Ca(2+) uptake and prolonged release from mitochondria as a novel regulatory mechanism in neuronal excitation-transcription coupling.
Figures




Similar articles
-
Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons.J Neurosci. 2008 May 14;28(20):5295-311. doi: 10.1523/JNEUROSCI.4810-07.2008. J Neurosci. 2008. PMID: 18480286 Free PMC article.
-
Distinct activation properties of the nuclear factor of activated T-cells (NFAT) isoforms NFATc3 and NFATc4 in neurons.J Biol Chem. 2012 Nov 2;287(45):37594-609. doi: 10.1074/jbc.M112.365197. Epub 2012 Sep 12. J Biol Chem. 2012. PMID: 22977251 Free PMC article.
-
Nerve growth factor (NGF) regulates activity of nuclear factor of activated T-cells (NFAT) in neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen synthase kinase 3β (GSK3β) pathway.J Biol Chem. 2014 Nov 7;289(45):31349-60. doi: 10.1074/jbc.M114.587188. Epub 2014 Sep 17. J Biol Chem. 2014. PMID: 25231981 Free PMC article.
-
Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes.Cell Calcium. 2007 Aug;42(2):145-56. doi: 10.1016/j.ceca.2007.03.007. Epub 2007 Jun 18. Cell Calcium. 2007. PMID: 17572487 Review.
-
TRP Channels in the Heart.In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 9. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 9. PMID: 29356479 Free Books & Documents. Review.
Cited by
-
Ca2+ Microdomains, Calcineurin and the Regulation of Gene Transcription.Cells. 2021 Apr 12;10(4):875. doi: 10.3390/cells10040875. Cells. 2021. PMID: 33921430 Free PMC article. Review.
-
Computational properties of mitochondria in T cell activation and fate.Open Biol. 2016 Nov;6(11):160192. doi: 10.1098/rsob.160192. Open Biol. 2016. PMID: 27852805 Free PMC article. Review.
-
Sensory nerve terminal mitochondrial dysfunction induces hyperexcitability in airway nociceptors via protein kinase C.Mol Pharmacol. 2014 Jun;85(6):839-48. doi: 10.1124/mol.113.091272. Epub 2014 Mar 18. Mol Pharmacol. 2014. PMID: 24642367 Free PMC article.
-
Mitochondrial calcium signalling and neurodegenerative diseases.Neuronal Signal. 2018 Nov 16;2(4):NS20180061. doi: 10.1042/NS20180061. eCollection 2018 Dec. Neuronal Signal. 2018. PMID: 32714593 Free PMC article. Review.
-
Agonist-dependence of functional properties for common nonsynonymous variants of human transient receptor potential vanilloid 1.Pain. 2016 Jul;157(7):1515-1524. doi: 10.1097/j.pain.0000000000000556. Pain. 2016. PMID: 26967694 Free PMC article.
References
-
- Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. - PubMed
-
- Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
-
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. - PubMed
-
- Baron KT, Thayer SA. CGP37157 modulates mitochondrial Ca2+ homeostasis in cultured rat dorsal root ganglion neurons. Eur J Pharmacol. 1997;340:295–300. - PubMed
-
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
