Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome
- PMID: 19793977
- PMCID: PMC2846656
- DOI: 10.1523/JNEUROSCI.3156-09.2009
Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome
Abstract
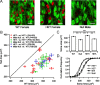
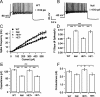
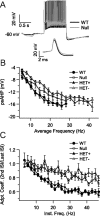
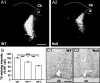
Rett syndrome (RTT) is a neurodevelopmental disorder caused by loss-of-function mutations in the Methyl-CpG-binding protein-2 (MECP2) gene and is characterized by derangements in cognition, behavior, motor control, respiration and autonomic homeostasis, as well as seizures. Deficits in norepinephrine (NE) are thought to contribute to RTT pathogenesis, but little is known about how MeCP2 regulates function of noradrenergic neurons. We therefore characterized morphological, electrical, and neurochemical properties of neurons in the locus ceruleus (LC), the major source of noradrenergic innervation to the central neuraxis, in Mecp2 mutant mice. We found that MeCP2 null LC neurons are electrically hyperexcitable, smaller in size, and express less of the NE-synthesizing enzyme tyrosine hydroxylase (TH) compared with wild-type neurons. Increased excitability of mutant neurons is associated with reductions in passive membrane conductance and the amplitude of the slow afterhyperpolarization. Studies in Mecp2 heterozygotes, which are mosaic for the null allele, demonstrated that electrical hyperexcitability and reduced neuronal size are cell-autonomous consequences of MeCP2 loss, whereas reduced TH expression appears to reflect both cell-autonomous and non-autonomous influences. Finally, we found reduced levels of TH and norepinephrine in cingulate cortex, a forebrain target of the LC. Thus, genetic loss of MeCP2 results in a somewhat paradoxical LC neuron phenotype, characterized by both electrical hyperexcitability and reduced indices of noradrenergic function. Given the importance of the LC in modulating activity in brainstem and forebrain networks, we hypothesize that dysregulation of LC function in the absence of MeCP2 plays a key role in the pathophysiology of RTT.
Figures
Similar articles
-
Effects of early-life exposure to THIP on brainstem neuronal excitability in the Mecp2-null mouse model of Rett syndrome before and after drug withdrawal.Physiol Rep. 2017 Jan;5(2):e13110. doi: 10.14814/phy2.13110. Physiol Rep. 2017. PMID: 28108647 Free PMC article.
-
An optogenetic mouse model of rett syndrome targeting on catecholaminergic neurons.J Neurosci Res. 2016 Oct;94(10):896-906. doi: 10.1002/jnr.23760. Epub 2016 Jun 18. J Neurosci Res. 2016. PMID: 27317352 Free PMC article.
-
Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome.Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C508-20. doi: 10.1152/ajpcell.00035.2014. Epub 2014 Jul 9. Am J Physiol Cell Physiol. 2014. PMID: 25009110 Free PMC article.
-
Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review.
-
Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development.Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644. Epub 2020 Jan 2. Brain Res. 2020. PMID: 31904347 Free PMC article. Review.
Cited by
-
Autism spectrum disorder: neuropathology and animal models.Acta Neuropathol. 2017 Oct;134(4):537-566. doi: 10.1007/s00401-017-1736-4. Epub 2017 Jun 5. Acta Neuropathol. 2017. PMID: 28584888 Free PMC article. Review.
-
Clinical presentation of Rett syndrome in relation to quality of life and family functioning.J Int Med Res. 2021 Apr;49(4):3000605211007714. doi: 10.1177/03000605211007714. J Int Med Res. 2021. PMID: 33906527 Free PMC article.
-
Reduced neuronal size and mTOR pathway activity in the Mecp2 A140V Rett syndrome mouse model.F1000Res. 2016 Sep 8;5:2269. doi: 10.12688/f1000research.8156.1. eCollection 2016. F1000Res. 2016. PMID: 27781091 Free PMC article.
-
Early Life Stress and Epigenetics in Late-onset Alzheimer's Dementia: A Systematic Review.Curr Genomics. 2018 Nov;19(7):522-602. doi: 10.2174/1389202919666171229145156. Curr Genomics. 2018. PMID: 30386171 Free PMC article. Review.
-
Genetic effects on longitudinal cognitive decline during the early stages of Alzheimer's disease.Sci Rep. 2021 Oct 6;11(1):19853. doi: 10.1038/s41598-021-99310-z. Sci Rep. 2021. PMID: 34615922 Free PMC article.
References
-
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–335. - PubMed
-
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. - PubMed
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. - PubMed
-
- Bauman ML, Kemper TL, Arin DM. Pervasive neuroanatomic abnormalities of the brain in three cases of Rett's syndrome. Neurology. 1995;45:1581–1586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
