Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats
- PMID: 19793979
- PMCID: PMC6666156
- DOI: 10.1523/JNEUROSCI.1828-09.2009
Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats
Abstract
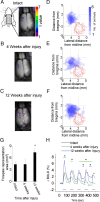
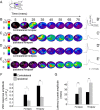
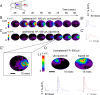
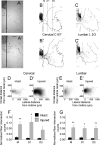
A lateral hemisection injury of the cervical spinal cord results in Brown-Séquard syndrome in humans and rats. The hands/forelimbs on the injured side are rendered permanently impaired, but the legs/hindlimbs recover locomotor functions. This is accompanied by increased use of the forelimb on the uninjured side. Nothing is known about the cortical circuits that correspond to these behavioral adaptations. In this study, on adult rats with cervical spinal cord lateral hemisection lesions (at segment C3/4), we explored the sensory representation and corticospinal projection of the intact (ipsilesional) cortex. Using blood oxygenation level-dependent functional magnetic resonance imaging and voltage-sensitive dye (VSD) imaging, we found that the cortex develops an enhanced representation of the unimpaired forepaw by 12 weeks after injury. VSD imaging also revealed the cortical spatio-temporal dynamics in response to electrical stimulation of the ipsilateral forepaw or hindpaw. Interestingly, stimulation of the ipsilesional hindpaw at 12 weeks showed a distinct activation of the hindlimb area in the intact, ipsilateral cortex, probably via the injury-spared spinothalamic pathway. Anterograde tracing of corticospinal axons from the intact cortex showed sprouting to recross the midline, innervating the spinal segments below the injury in both cervical and lumbar segments. Retrograde tracing of these midline-crossing axons from the cervical spinal cord (at segment C6/7) revealed the formation of a new ipsilateral forelimb representation in the cortex. Our results demonstrate profound reorganizations of the intact sensory-motor cortex after unilateral spinal cord injury. These changes may contribute to the behavioral adaptations, notably for the recovery of the ipsilesional hindlimb.
Figures


References
-
- Akintunde A, Buxton DF. Differential sites of origin and collateralization of corticospinal neurons in the rat: a multiple fluorescent retrograde tracer study. Brain Res. 1992;575:86–92. - PubMed
-
- Bolton DA, Tse AD, Ballermann M, Misiaszek JE, Fouad K. Task specific adaptations in rat locomotion: runway versus horizontal ladder. Behav Brain Res. 2006;168:272–279. - PubMed
-
- Brown-Séquard CE. Lectures on the physiology and pathology of the nervous system; and on the treatment of organic nervous affections. Lancet. 1868;92:593.
-
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10:3918–3922. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
