Characteristics of Candida albicans biofilms grown in a synthetic urine medium
- PMID: 19794044
- PMCID: PMC2786631
- DOI: 10.1128/JCM.01377-09
Characteristics of Candida albicans biofilms grown in a synthetic urine medium
Abstract
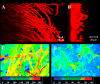
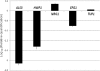
Urinary tract infections (UTIs) are the most common type of nosocomial infection, and Candida albicans is the most frequent organism causing fungal UTIs. Presence of an indwelling urinary catheter represents a significant risk factor for UTIs. Furthermore, these infections are frequently associated with the formation of biofilms on the surface of these catheters. Here, we describe the characterization of C. albicans biofilms formed in vitro using synthetic urine (SU) medium and the frequently used RPMI medium and compare the results. Biofilms of C. albicans strain SC5314 were formed in 96-well microtiter plates and on silicon elastomer pieces using both SU and RPMI media. Biofilm formation was monitored by microscopy and a colorimetric XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reduction assay. As in biofilms grown in RPMI medium, time course studies revealed that biofilm formation using SU medium occurred after an initial adherence phase, followed by growth, proliferation, and maturation. However, microscopy techniques revealed that the architectural complexity of biofilms formed in SU medium was lower than that observed for those formed using RPMI medium. In particular, the level of filamentation of cells within the biofilms formed in SU medium was diminished compared to those in the biofilms grown in RPMI medium. This observation was also corroborated by expression profiling of five filamentation-associated genes using quantitative real-time reverse transcriptase PCR. Sessile C. albicans cells were resistant to fluconazole and amphotericin B, irrespective of the medium used to form the biofilms. However, caspofungin exhibited potent in vitro activity at therapeutic levels against C. albicans biofilms grown in both SU and RPMI media.
Figures



References
-
- Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. - PubMed
-
- Cocuaud, C., M. H. Rodier, G. Daniault, and C. Imbert. 2005. Anti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilms. J. Antimicrob. Chemother. 56:507-512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

