4.1R-deficient human red blood cells have altered phosphatidylserine exposure pathways and are deficient in CD44 and CD47 glycoproteins
- PMID: 19794081
- PMCID: PMC2754950
- DOI: 10.3324/haematol.2009.006585
4.1R-deficient human red blood cells have altered phosphatidylserine exposure pathways and are deficient in CD44 and CD47 glycoproteins
Abstract
Background: Protein 4.1R is an important component of the red cell membrane skeleton. It imparts structural integrity and has transmembrane signaling roles by direct interactions with transmembrane proteins and other membrane skeletal components, notably p55 and calmodulin.
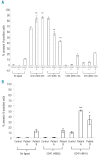
Design and methods: Spontaneous and ligation-induced phosphatidylserine exposure on erythrocytes from two patients with 4.1R deficiency were studied, using CD47 glycoprotein and glycophorin C as ligands. We also looked for protein abnormalities in the 4.1R-based multiprotein complex.
Results: Phosphatidylserine exposure was significantly increased in 4.1R-deficient erythrocytes obtained from the two different individuals when ligands to CD47 glycoprotein were bound. Spontaneous phosphatidylserine exposure was normal. 4.1R, glycophorin C and p55 were missing or sharply reduced. Furthermore there was an alteration or deficiency of CD47 glycoprotein and a lack of CD44 glycoprotein. Based on a recent study in 4.1R-deficient mice, we found that there are clear functional differences between interactions of human red cell 4.1R and its murine counterpart.
Conclusions: Glycophorin C is known to bind 4.1R, and we have defined previously that it also binds CD47. From our evidence, we suggest that 4.1R plays a role in the phosphatidylserine exposure signaling pathway that is of fundamental importance in red cell turnover. The linkage of CD44 to 4.1R may be relevant to this process.
Figures


References
-
- Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1–20. - PubMed
-
- An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–75. - PubMed
-
- Marfatia SM, Morais-Cabral JH, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. Analysis of the binding interface by in vitro mutagenesis. J Biol Chem. 1997;272:24191–7. - PubMed
-
- Marfatia SM, Leu RA, Branton D, Chishti AH. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J Biol Chem. 1995;270:715–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

