A new function for parietal epithelial cells: a second glomerular barrier
- PMID: 19794110
- PMCID: PMC2801333
- DOI: 10.1152/ajprenal.00214.2009
A new function for parietal epithelial cells: a second glomerular barrier
Abstract
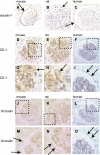
The functional role of glomerular parietal epithelial cells (PECs) remains poorly understood. To test the hypothesis that PECs form an impermeable barrier to filtered protein through the formation of tight junctions (TJ), studies were performed in normal animals and in the anti-glomerular basement membrane (GBM) model of crescentic nephritis. Electron microscopy showed well-defined TJ between PECs in normal mice, which no longer could be identified when these cells became extensively damaged or detached from their underlying Bowman's basement membrane. The TJ proteins claudin-1, zonula occludens-1, and occludin stained positive in PECs; however, staining decreased in anti-GBM disease. To show that these events were associated with increased permeability across the PEC-Bowman's basement membrane barrier, control and diseased animals were injected intravenously with either Texas red-conjugated dextran (3 kDa) or ovalbumin (45 kDa) tracers. As expected, both tracers were readily filtered across the glomerular filtration barrier and taken up by proximal tubular cells. However, when the glomerular filtration barrier was injured in anti-GBM disease, tracers were taken up by podocytes and PECs. Moreover, tracers were also detected between PECs and the underlying Bowman's basement membrane, and in many instances were detected in the extraglomerular space. We propose that together with its underlying Bowman's basement membrane, the TJ of PECs serve as a second barrier to protein. When disturbed following PEC injury, the increase in permeability of this layer to filtered protein is a mechanism underlying periglomerular inflammation characteristic of anti-GBM disease.
Figures






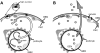
References
-
- Atkins RC, Nikolic-Paterson DJ, Song Q, Lan HY. Modulators of crescentic glomerulonephritis. J Am Soc Nephrol 7: 2271–2278, 1996 - PubMed
-
- Barisoni L, Nelson PJ. Collapsing glomerulopathy: an inflammatory podocytopathy? Curr Opin Nephrol Hypertens 16: 192–195, 2007 - PubMed
-
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003 - PubMed
-
- Griffin SV, Krofft RD, Pippin JW, Shankland SJ. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int 67: 977–986, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

