Mechanical stimuli modulate lateral root organogenesis
- PMID: 19794120
- PMCID: PMC2785988
- DOI: 10.1104/pp.109.142448
Mechanical stimuli modulate lateral root organogenesis
Abstract
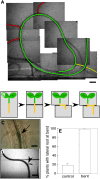
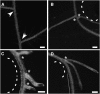
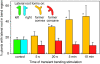
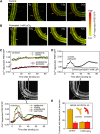
Unlike mammals, whose development is limited to a short temporal window, plants produce organs de novo throughout their lifetime in order to adapt their architecture to the prevailing environmental conditions. The production of lateral roots represents one example of such postembryonic organogenesis. An endogenous developmental program likely imposes an ordered arrangement on the position of new lateral roots. However, environmental stimuli such as nutrient levels also affect the patterning of lateral root production. In addition, we have found that mechanical forces can act as one of the triggers that entrain lateral root production to the environment of the Arabidopsis (Arabidopsis thaliana) plant. We observed that physical bending of the root recruited new lateral root formation to the convex side of the resultant bend. Transient bending of 20 s was sufficient to elicit this developmental program. Such bending triggered a Ca(2+) transient within the pericycle, and blocking this change in Ca(2+) also blocked recruitment of new lateral root production to the curved region of the root. The initial establishment of the mechanically induced lateral root primordium was independent of an auxin supply from the shoot and was not disrupted by mutants in a suite of auxin transporters and receptor/response elements. These results suggest that Ca(2+) may be acting to translate the mechanical forces inherent in growth to a developmental response in roots.
Figures




References
-
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 - PubMed
-
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 39–44 - PubMed
-
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8 165–171 - PubMed
-
- De Smet I, Tetsumura T, De Rybel B, Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134 681–690 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

