In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance
- PMID: 19794140
- PMCID: PMC2792211
- DOI: 10.1182/blood-2009-04-214569
In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance
Abstract
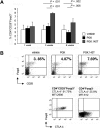
We previously showed that incorporating target sequences for the hematopoietic-specific microRNA miR-142 into an antigen-encoding transgene prevents antigen expression in antigen-presenting cells (APCs). To determine whether this approach induces immunologic tolerance, we treated mice with a miR-142-regulated lentiviral vector encoding green fluorescent protein (GFP), and subsequently vaccinated the mice against GFP. In contrast to control mice, no anti-GFP response was observed, indicating that robust tolerance to the transgene-encoded antigen was achieved. Furthermore, injection of the miR-142-regulated vector induced a population of GFP-specific regulatory T cells. Interestingly, an anti-GFP response was observed when microRNA miR-122a was inserted into the vector and antigen expression was detargeted from hepatocytes as well as APCs. This demonstrates that, in the context of lentiviral vector-mediated gene transfer, detargeting antigen expression from professional APCs, coupled with expression in hepatocytes, can induce antigen-specific immunologic tolerance.
Figures





References
-
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. - PubMed
-
- Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. - PubMed
-
- Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

