mTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-independent mechanisms
- PMID: 19794149
- PMCID: PMC2793064
- DOI: 10.1152/ajpcell.00248.2009
mTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-independent mechanisms
Abstract
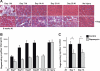
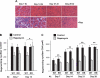
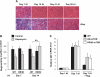
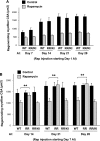
Rapamycin-sensitive signaling is required for skeletal muscle differentiation and remodeling. In cultured myoblasts, the mammalian target of rapamycin (mTOR) has been reported to regulate differentiation at different stages through distinct mechanisms, including one that is independent of mTOR kinase activity. However, the kinase-independent function of mTOR remains controversial, and no in vivo studies have examined those mTOR myogenic mechanisms previously identified in vitro. In this study, we find that rapamycin impairs injury-induced muscle regeneration. To validate the role of mTOR with genetic evidence and to probe the mechanism of mTOR function, we have generated and characterized transgenic mice expressing two mutants of mTOR under the control of human skeletal actin (HSA) promoter: rapamycin-resistant (RR) and RR/kinase-inactive (RR/KI). Our results show that muscle regeneration in rapamycin-administered mice is restored by RR-mTOR expression. In the RR/KI-mTOR mice, nascent myofiber formation during the early phase of regeneration proceeds in the presence of rapamycin, but growth of the regenerating myofibers is blocked by rapamycin. Igf2 mRNA levels increase drastically during early regeneration, which is sensitive to rapamycin in wild-type muscles but partially resistant to rapamycin in both RR- and RR/KI-mTOR muscles, consistent with mTOR regulation of Igf2 expression in a kinase-independent manner. Furthermore, systemic ablation of S6K1, a target of mTOR kinase, results in impaired muscle growth but normal nascent myofiber formation during regeneration. Therefore, mTOR regulates muscle regeneration through kinase-independent and kinase-dependent mechanisms at the stages of nascent myofiber formation and myofiber growth, respectively.
Figures



References
-
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol 14: 483– 510, 1996 - PubMed
-
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411– 424, 2008 - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014– 1019, 2001 - PubMed
-
- Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol 16: 225– 238, 1990 - PubMed
-
- Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol 268: 358– 371, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

