Pharmacological uncoupling of activation induced increases in CBF and CMRO2
- PMID: 19794398
- PMCID: PMC2949119
- DOI: 10.1038/jcbfm.2009.211
Pharmacological uncoupling of activation induced increases in CBF and CMRO2
Abstract
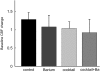
Neurovascular coupling provides the basis for many functional neuroimaging techniques. Nitric oxide (NO), adenosine, cyclooxygenase, CYP450 epoxygenase, and potassium are involved in dilating arterioles during neuronal activation. We combined inhibition of NO synthase, cyclooxygenase, adenosine receptors, CYP450 epoxygenase, and inward rectifier potassium (Kir) channels to test whether these pathways could explain the blood flow response to neuronal activation. Cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO(2)) of the somatosensory cortex were measured during forepaw stimulation in 24 rats using a laser Doppler/spectroscopy probe through a cranial window. Combined inhibition reduced CBF responses by two-thirds, somatosensory evoked potentials and activation-induced CMRO(2) increases remained unchanged, and deoxy-hemoglobin (deoxy-Hb) response was abrogated. This shows that in the rat somatosensory cortex, one-third of the physiological blood flow increase is sufficient to prevent microcirculatory increase of deoxy-Hb concentration during neuronal activity. The large physiological CBF response is not necessary to support small changes in CMRO(2). We speculate that the CBF response safeguards substrate delivery during functional activation with a considerable 'safety factor'. Reduction of the CBF response in pathological states may abolish the BOLD-fMRI signal, without affecting underlying neuronal activity.
Figures





References
-
- Bakalova R, Matsuura T, Kanno I. The cyclooxygenase inhibitors indomethacin and rofecoxib reduce regional cerebral blood flow evoked by somatosensory stimulation in rats. Exp Med Biol (Maywood) 2002;227:465–473. - PubMed
-
- Burke M, Bührle C.2006BOLD response during uncoupling of neuronal activity and CBF Neuroimage 321–8.e-pub 2006 May 3 - PubMed
-
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural activation. J Cereb Blood Flow Metab. 1997;17:64–72. - PubMed
-
- Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol. 1994;267 (1 Pt 2:H296–H301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

