Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression
- PMID: 19794406
- PMCID: PMC2795057
- DOI: 10.1038/npp.2009.149
Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression
Abstract
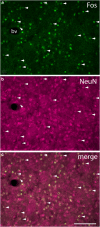
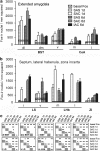
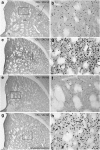
The effects of addictive psychostimulant drugs on the brain change over repeated administrations. We evaluated a large sample of brain structures, particularly ones comprising basal forebrain macrosystems, and determined in which the immediate-early gene product, Fos, is expressed following a single and repeated self-administrations of cocaine. The caudate-putamen and accumbens, comprising the basal ganglia input structures, and the hypothalamic supraoptic and paraventricular nuclei, lateral and medial habenula, mesopontine rostromedial tegmental nucleus and anterior cingulate cortex exhibited Fos expression enhanced by acute self-administration of cocaine (SAC), but desensitized after repeated administrations. Fos expression was mainly enhanced by acutely self-administered cocaine in basal ganglia output and intrinsic structures and the intermediate nucleus of lateral septum, medial division of the central amygdaloid nucleus and zona incerta, but, in contrast, was sensitized in these structures after repeated administrations. Acute and repeated SAC left Fos expression unaffected or marginally enhanced in most extended amygdala structures, of which nearly all, however, exhibited robustly increased Fos expression after repeated saline self-administration, occasionally to levels exceeding those elicited by cocaine. Thus, self-administered cocaine mainly elicits Fos expression, which persists or increases with repeated administrations in some structures, but declines in others. In addition, Fos expression is sensitized in most extended amygdala structures merely by the act of repeated self-administering. Similar spatiotemporal patterns of cocaine- or saline-elicited Fos expression characterize functionally related clusters of structures, such as, eg, basal ganglia input structures, basal ganglia output structures, extended amygdala and structures in the brainstem to which forebrain macrosystems project.
Figures








Similar articles
-
Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats.J Neurosci. 2012 Sep 19;32(38):13309-26. doi: 10.1523/JNEUROSCI.2277-12.2012. J Neurosci. 2012. PMID: 22993446 Free PMC article.
-
Fos expression induced by cocaine-conditioned cues in male and female rats.Brain Struct Funct. 2014 Sep;219(5):1831-40. doi: 10.1007/s00429-013-0605-8. Epub 2013 Jul 6. Brain Struct Funct. 2014. PMID: 23832598 Free PMC article.
-
Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues.Eur Neuropsychopharmacol. 2008 Aug;18(8):600-11. doi: 10.1016/j.euroneuro.2008.04.010. Epub 2008 Jun 6. Eur Neuropsychopharmacol. 2008. PMID: 18539009 Free PMC article.
-
Persistent activation of select forebrain regions in aggressive, adolescent cocaine-treated hamsters.Behav Brain Res. 2005 Apr 30;159(2):277-86. doi: 10.1016/j.bbr.2004.11.027. Epub 2005 Jan 6. Behav Brain Res. 2005. PMID: 15817190
-
Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression.Neuroscience. 2010 Dec 29;171(4):1187-96. doi: 10.1016/j.neuroscience.2010.10.001. Epub 2010 Oct 8. Neuroscience. 2010. PMID: 20933585 Free PMC article.
Cited by
-
Lesions of the fasciculus retroflexus alter footshock-induced cFos expression in the mesopontine rostromedial tegmental area of rats.PLoS One. 2013 Apr 12;8(4):e60678. doi: 10.1371/journal.pone.0060678. Print 2013. PLoS One. 2013. PMID: 23593280 Free PMC article.
-
Routing of Hippocampal Ripples to Subcortical Structures via the Lateral Septum.Neuron. 2020 Jan 8;105(1):138-149.e5. doi: 10.1016/j.neuron.2019.10.012. Epub 2019 Nov 26. Neuron. 2020. PMID: 31784288 Free PMC article.
-
Whole-brain tracking of cocaine and sugar rewards processing.Transl Psychiatry. 2023 Jan 23;13(1):20. doi: 10.1038/s41398-023-02318-4. Transl Psychiatry. 2023. PMID: 36683039 Free PMC article.
-
The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors.Prog Neurobiol. 2015 Jul;130:29-70. doi: 10.1016/j.pneurobio.2015.03.005. Epub 2015 Apr 6. Prog Neurobiol. 2015. PMID: 25857550 Free PMC article. Review.
-
Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons.Neuropharmacology. 2013 Jul;70:180-9. doi: 10.1016/j.neuropharm.2013.01.008. Epub 2013 Jan 21. Neuropharmacology. 2013. PMID: 23347950 Free PMC article.
References
-
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. - PubMed
-
- Asmus SE, Newman SW. Colocalization of tyrosine hydroxylase and Fos in the male Syrian hamster brain following different states of arousal. J Neurobiol. 1994;25:156–168. - PubMed
-
- Balster RL. Abuse potential of buspirone and related drugs. J Clin Psychopharmacol. 1990;10:31S–37S. - PubMed
-
- Bauer LO, Kranzler HR. Electroencephalographic activity and mood in cocaine-dependent outpatients: effects of cocaine cue exposure. Biol Psychiat. 1994;36:189–197. - PubMed
-
- Bekavac I, Waterhouse BD. Systemically administered cocaine selectively enhances long-latency responses of rat barrel field cortical neurons to vibrissae stimulation. J Pharmacol Exp Ther. 1995;272:333–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

