Adipose tissue-organotypic culture system as a promising model for studying adipose tissue biology and regeneration
- PMID: 19794899
- PMCID: PMC2710525
- DOI: 10.4161/org.5.2.8347
Adipose tissue-organotypic culture system as a promising model for studying adipose tissue biology and regeneration
Abstract
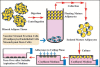
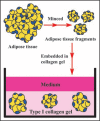
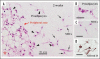
Adipose tissue consists of mature adipocytes, preadipocytes and mesenchymal stem cells (MSCs), but a culture system for analyzing their cell types within the tissue has not been established. We have recently developed "adipose tissue-organotypic culture system" that maintains unilocular structure, proliferative ability and functions of mature adipocytes for a long term, using three-dimensional collagen gel culture of the tissue fragments. In this system, both preadipocytes and MSCs regenerate actively at the peripheral zone of the fragments. Our method will open up a new way for studying both multiple cell types within adipose tissue and the cell-based mechanisms of obesity and metabolic syndrome. Thus, it seems to be a promising model for investigating adipose tissue biology and regeneration. In this article, we introduce adipose tissue-organotypic culture, and propose two theories regarding the mechanism of tissue regeneration that occurs specifically at peripheral zone of tissue fragments in vitro.
Keywords: adipokines; adipose tissue-organotypic culture; central zone; mature adipocytes; mesenchymal stem cells; peripheral zone; preadipocytes (immature adipocytes); three-dimensional; tissue fragments; tissue regeneration.
Figures





Similar articles
-
A new organotypic culture of adipose tissue fragments maintains viable mature adipocytes for a long term, together with development of immature adipocytes and mesenchymal stem cell-like cells.Endocrinology. 2008 Oct;149(10):4794-8. doi: 10.1210/en.2008-0525. Epub 2008 Jun 5. Endocrinology. 2008. PMID: 18535101
-
3D collagen microfibers stimulate the functionality of preadipocytes and maintain the phenotype of mature adipocytes for long term cultures.Acta Biomater. 2019 Jan 15;84:194-207. doi: 10.1016/j.actbio.2018.11.048. Epub 2018 Nov 28. Acta Biomater. 2019. PMID: 30502481
-
Adipose progenitor cells reside among the mature adipocytes: morphological research using an organotypic culture system.Cell Biol Int. 2015 Nov;39(11):1288-98. doi: 10.1002/cbin.10503. Epub 2015 Jul 22. Cell Biol Int. 2015. PMID: 26095163
-
Hypoxia and adipose tissue function and dysfunction in obesity.Physiol Rev. 2013 Jan;93(1):1-21. doi: 10.1152/physrev.00017.2012. Physiol Rev. 2013. PMID: 23303904 Review.
-
Cellular models for understanding adipogenesis, adipose dysfunction, and obesity.J Cell Biochem. 2010 Jun 1;110(3):564-72. doi: 10.1002/jcb.22598. J Cell Biochem. 2010. PMID: 20512917 Review.
Cited by
-
Culture models for studying thyroid biology and disorders.ISRN Endocrinol. 2011;2011:275782. doi: 10.5402/2011/275782. Epub 2011 Jul 12. ISRN Endocrinol. 2011. PMID: 22363871 Free PMC article.
-
Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro.J Exp Clin Cancer Res. 2012 Apr 2;31(1):32. doi: 10.1186/1756-9966-31-32. J Exp Clin Cancer Res. 2012. PMID: 22469146 Free PMC article.
-
Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles.Tissue Eng Part C Methods. 2013 May;19(5):336-44. doi: 10.1089/ten.TEC.2012.0198. Epub 2012 Nov 2. Tissue Eng Part C Methods. 2013. PMID: 23017116 Free PMC article.
-
Building invitro 3D human multicellular models of high-grade serous ovarian cancer.STAR Protoc. 2022 Jan 11;3(1):101086. doi: 10.1016/j.xpro.2021.101086. eCollection 2022 Mar 18. STAR Protoc. 2022. PMID: 35072115 Free PMC article.
-
Effects of vitamin A on in vitro maturation of pre-pubertal mouse spermatogonial stem cells.PLoS One. 2013 Dec 9;8(12):e82819. doi: 10.1371/journal.pone.0082819. eCollection 2013. PLoS One. 2013. PMID: 24349372 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
