Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice
- PMID: 19794969
- PMCID: PMC2745676
- DOI: 10.1593/neo.09934
Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice
Abstract
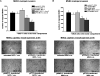
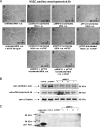
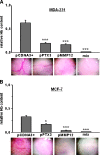
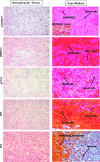
We have previously shown that endothelial cell matrix metalloprotease 12 (MMP12) and pentraxin 3 (PTX3) overproduction is the main alteration accounting for reduced proneness to angiogenesis in systemic sclerosis (SSc). On this basis, we stably transfected MMP12 and PTX3 in two breast cancer cell lines expressing very low amounts of the target molecules when compared with normal breast epithelial cells, relying on the hypothesis that antiangiogenic molecules released by cancer cells could confer an SSc-like antiangiogenic pattern on target endothelial cells. In Matrigel Boyden chamber invasion and capillary morphogenesis studies, transfected clones reduced endothelial cell invasion and capillary tube formation, which were abolished by tumor cell populations expressing both molecules. The Matrigel sponge assay, performed in vivo in C57/BL6 mice by injecting aliquots of lyophilized culture medium of transfected clones, indicated a similar reduction in angiogenesis. Functional studies have shown that endothelial cells treated with a culture medium of MMP12-expressing clones underwent cleavage of urokinase-type plasminogen activator receptor domain 1 which is indispensable to angiogenesis. We did not observe angiostatin production from plasminogen under the same experimental conditions. PTX3-overexpressing clones showed a powerful anti-fibroblast growth factor 2 (FGF2) activity in FGF2-dependent capillary morphogenesis. We have injected control and transfected clones into nude nu/nu (CD-1) BR mice to study the differential tumor growth pattern. We observed a reduction of tumor growth in transfected clones, which was basically complete when clones expressing both molecules were simultaneously injected. The extent of tumor necrosis suggested an antiangiogenesis-dependent inhibition of tumor development.
Figures



References
-
- Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69:14–16. - PubMed
-
- Carmeliet P, Collen D. Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol. 2000;190:387–405. - PubMed
-
- Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. - PubMed
-
- Maxwell PH. The HIF pathway in cancer (2005) Semin Cell Dev Biol. 2005;16:523–530. - PubMed
-
- Cutolo M, Grassi W, Matucci-Cerinic M. Raynaud's phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003;48:3023–3030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
