Rapid assessment of internodal myelin integrity in central nervous system tissue
- PMID: 19795370
- PMCID: PMC2813316
- DOI: 10.1002/jnr.22241
Rapid assessment of internodal myelin integrity in central nervous system tissue
Abstract
Monitoring pathology/regeneration in experimental models of de-/remyelination requires an accurate measure not only of functional changes but also of the amount of myelin. We tested whether X-ray diffraction (XRD), which measures periodicity in unfixed myelin, can assess the structural integrity of myelin in fixed tissue. From laboratories involved in spinal cord injury research and in studying the aging primate brain, we solicited "blind" samples and used an electronic detector to record rapidly the diffraction patterns (30 min each pattern) from them. We assessed myelin integrity by measuring its periodicity and relative amount. Fixation of tissue itself introduced +/-10% variation in periodicity and +/-40% variation in relative amount of myelin. For samples having the most native-like periods, the relative amounts of myelin detected allowed distinctions to be made between normal and demyelinating segments, between motor and sensory tracts within the spinal cord, and between aged and young primate CNS. Different periodicities also allowed distinctions to be made between samples from spinal cord and nerve roots and between well-fixed and poorly fixed samples. Our findings suggest that, in addition to evaluating the effectiveness of different fixatives, XRD could also be used as a robust and rapid technique for quantitating the relative amount of myelin among spinal cords and other CNS tissue samples from experimental models of de- and remyelination.
Figures

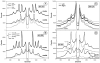

Similar articles
-
Internodal myelination during development quantitated using X-ray diffraction.J Struct Biol. 2009 Dec;168(3):521-6. doi: 10.1016/j.jsb.2009.06.019. Epub 2009 Jul 1. J Struct Biol. 2009. PMID: 19576284
-
miR-219 Cooperates with miR-338 in Myelination and Promotes Myelin Repair in the CNS.Dev Cell. 2017 Mar 27;40(6):566-582.e5. doi: 10.1016/j.devcel.2017.03.001. Dev Cell. 2017. PMID: 28350989 Free PMC article.
-
Changes in the distribution of a calcium-dependent ATPase during demyelination and remyelination in the central nervous system.J Neurocytol. 1996 Mar;25(3):171-80. doi: 10.1007/BF02284794. J Neurocytol. 1996. PMID: 8737170
-
Remyelination in demyelinating diseases of the central nervous system.Crit Rev Neurobiol. 1987;3(1):1-28. Crit Rev Neurobiol. 1987. PMID: 3552450 Review.
-
Remyelination in the CNS: from biology to therapy.Nat Rev Neurosci. 2008 Nov;9(11):839-55. doi: 10.1038/nrn2480. Nat Rev Neurosci. 2008. PMID: 18931697 Review.
Cited by
-
Metabolically-incorporated deuterium in myelin localized by neutron diffraction and identified by mass spectrometry.Curr Res Struct Biol. 2022 Jun 28;4:231-245. doi: 10.1016/j.crstbi.2022.06.003. eCollection 2022. Curr Res Struct Biol. 2022. PMID: 35941866 Free PMC article.
-
Myelin abnormalities in the optic and sciatic nerves in mice with GM1-gangliosidosis.ASN Neuro. 2015 Feb 18;7(1):1759091415568913. doi: 10.1177/1759091415568913. Print 2015 Jan-Feb. ASN Neuro. 2015. PMID: 25694553 Free PMC article.
References
-
- Agrawal D, Hawk R, Avila RL, Inouye H, Kirschner DA. Internodal myelination during development quantitated using x-ray diffraction. J Struct Biol. 2009 accepted. - PubMed
-
- Avila RL. Ph.D. Dissertation. Chestnut Hill, MA: Boston College; 2007. Analysis of Internodal Myelin Structure in Transgenic Mice and Zebrafish Using X-Ray Diffraction; p. 286.
-
- Avila RL, Inouye H, Baek R, Yin X, Trapp BD, Feltri ML, Wrabetz L, Kirschner DA. Structure and stability of internodal myelin in mouse models of hereditary neuropathy. J Neuropathol Exp Neurol. 2005;64:976–990. - PubMed
-
- Blakemore WF, Crang AJ. The relationship between type-1 astrocytes, Schwann cells and oligodendrocytes following transplantation of glial cell cultures into demyelinating lesions in the adult rat spinal cord. J Neurocytol. 1989;18(4):519–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

