Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma
- PMID: 19795457
- PMCID: PMC2830344
- DOI: 10.1002/ijc.24909
Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma
Abstract
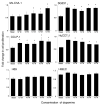
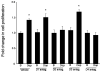
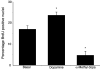
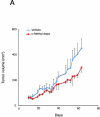
Cholangiocarcinoma is a devastating cancer of biliary origin with limited treatment options. Symptoms are usually evident after blockage of the bile duct by the tumor, and at this late stage, they are relatively resistant to chemotherapy and radiation therapy. Therefore, it is imperative that alternative treatment options are explored. We have previously shown that serotonin metabolism is dysregulated in cholangiocarcinoma leading to an increased secretion of serotonin, which has growth-promoting effects. Because serotonin and dopamine share the degradation machinery, we evaluated the secretion of dopamine from cholangiocarcinoma and its effects on cell proliferation. Using 4 cholangiocarcinoma cell lines and human biopsy samples, we demonstrated that there was an increase in mRNA and protein expression of the dopamine synthesis enzymes tyrosine hydroxylase and dopa decarboxylase in cholangiocarcinoma. There was increased dopamine secretion from cholangiocarcinoma cell lines compared to H69 and HIBEC cholangiocytes and increased dopamine immunoreactivity in human biopsy samples. Furthermore, administration of dopamine to all cholangiocarcinoma cell lines studied increased proliferation by up to 30%, which could be blocked by the pretreatment of the D2 and D4 dopamine receptor antagonists, whereas blocking dopamine production by alpha-methyldopa administration suppressed growth by up to 25%. Administration of alpha-methyldopa to nude mice also suppressed cholangiocarcinoma tumor growth. The data presented here represent the first evidence that dopamine metabolism is dysregulated in cholangiocarcinoma and that modulation of dopamine synthesis may represent an alternative target for the development of therapeutic strategies.
Figures









Similar articles
-
Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth.Cancer Res. 2008 Nov 15;68(22):9184-93. doi: 10.1158/0008-5472.CAN-08-2133. Cancer Res. 2008. PMID: 19010890 Free PMC article.
-
Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma.Gut. 2012 May;61(5):753-64. doi: 10.1136/gutjnl-2011-300007. Epub 2011 Aug 26. Gut. 2012. PMID: 21873469 Free PMC article.
-
Neuropeptide Y inhibits cholangiocarcinoma cell growth and invasion.Am J Physiol Cell Physiol. 2011 May;300(5):C1078-89. doi: 10.1152/ajpcell.00358.2010. Epub 2011 Jan 26. Am J Physiol Cell Physiol. 2011. PMID: 21270292 Free PMC article.
-
Mechanisms of biliary carcinogenesis and growth.World J Gastroenterol. 2008 May 21;14(19):2986-9. doi: 10.3748/wjg.14.2986. World J Gastroenterol. 2008. PMID: 18494047 Free PMC article. Review.
-
Recent advances in the regulation of cholangiocarcinoma growth.Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G1-9. doi: 10.1152/ajpgi.00114.2010. Epub 2010 Apr 29. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20430870 Free PMC article. Review.
Cited by
-
Correlation between Neurotransmitters (Dopamine, Epinephrine, Norepinephrine, Serotonin), Prognostic Nutritional Index, Glasgow Prognostic Score, Systemic Inflammatory Response Markers, and TNM Staging in a Cohort of Colorectal Neuroendocrine Tumor Patients.Int J Mol Sci. 2024 Jun 26;25(13):6977. doi: 10.3390/ijms25136977. Int J Mol Sci. 2024. PMID: 39000088 Free PMC article.
-
Cholangiocarcinoma: novel therapeutic targets.Expert Opin Ther Targets. 2020 Apr;24(4):345-357. doi: 10.1080/14728222.2020.1733528. Epub 2020 Feb 26. Expert Opin Ther Targets. 2020. PMID: 32077341 Free PMC article. Review.
-
Ferroptosis-related genes are potential prognostic molecular markers for patients with colorectal cancer.Clin Exp Med. 2021 Aug;21(3):467-477. doi: 10.1007/s10238-021-00697-w. Epub 2021 Mar 6. Clin Exp Med. 2021. PMID: 33674956
-
Research progress on the roles of dopamine and dopamine receptors in digestive system diseases.J Cell Mol Med. 2024 Apr;28(7):e18154. doi: 10.1111/jcmm.18154. J Cell Mol Med. 2024. PMID: 38494840 Free PMC article. Review.
-
Resveratrol enhances the sensitivity of cholangiocarcinoma to chemotherapeutic agents.Lab Invest. 2010 Sep;90(9):1325-38. doi: 10.1038/labinvest.2010.99. Epub 2010 May 10. Lab Invest. 2010. PMID: 20458282 Free PMC article.
References
-
- Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–9. - PubMed
-
- Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–50. ix. - PubMed
-
- Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

