6-Azahemiporphycene: a new member of the porphyrinoid family
- PMID: 19795835
- PMCID: PMC2793671
- DOI: 10.1021/ic9014866
6-Azahemiporphycene: a new member of the porphyrinoid family
Abstract
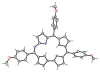
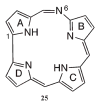
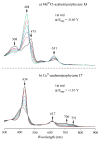
The reaction of 5,10,15-triarylcorrole with 4-amino-4H-1,2,4-triazole provides another example of corrole ring expansion to give the corresponding 6-azahemiporphycene, a novel porphyrin analogue. The facile oxidation of the corrole ring is a required step for the ring expansion and for this reason the reaction fails in the case of corroles bearing meso-phenyl groups carrying electron-withdrawing substituents. Steric requirements also limited the scope of the reaction, which is not successful in the case of 2,6-disubstituted meso-aryl corroles. The occurrence of an initial oxidation is further supported by formation of the 6-azahemiporphycene derivative when the reaction is carried out under the same conditions, using a 5- or a 10-isocorrole as starting material. (1)H NMR spectra and X-ray crystal characterization of 6-azahemiporphycene evidenced the presence of an intramolecular N-H...N hydrogen bond in the inner core of the macrocycle, while photophysical characterization confirmed the aromatic character of the novel macrocycle, showing an intense Soret-like band around 410 nm in the absorption spectrum. The fluorescence emission is very modest, and 6-azahemiporphycene showed higher photostability than the corresponding corrole species. Different metal complexes of 6-azahemiporphycene were prepared following synthetic protocols usually exploited for the preparation of metalloporphyrins, demonstrating good coordination properties for the macrocycle. Both the free-base and metal derivatives were characterized by cyclic voltammetry and spectroelectrochemistry in dichloromethane and benzonitrile. To further detail the behavior of this novel macrocycle, density functional theory (DFT) calculations were carried out on the basic structure of 6-azahemiporphycene with the aim of assessing aromaticity and tautomerism, as well as calculating its stability with respect to the 5-aza isomer.
Figures







Similar articles
-
Electronic absorption, resonance Raman, and electrochemical studies of planar and saddled copper(III) meso-triarylcorroles. Highly substituent-sensitive Soret bands as a distinctive feature of high-valent transition metal corroles.J Am Chem Soc. 2002 Jul 10;124(27):8104-16. doi: 10.1021/ja0113697. J Am Chem Soc. 2002. PMID: 12095356
-
β-Nitro-5,10,15-tritolylcorroles.Inorg Chem. 2012 Jun 18;51(12):6928-42. doi: 10.1021/ic3007926. Epub 2012 Jun 5. Inorg Chem. 2012. PMID: 22668242 Free PMC article.
-
The structural chemistry of metallocorroles: combined X-ray crystallography and quantum chemistry studies afford unique insights.Acc Chem Res. 2012 Aug 21;45(8):1203-14. doi: 10.1021/ar200292d. Epub 2012 Mar 23. Acc Chem Res. 2012. PMID: 22444488
-
Synthesis and functionalization of meso-aryl-substituted corroles.J Org Chem. 2001 Jan 26;66(2):550-6. doi: 10.1021/jo005661t. J Org Chem. 2001. PMID: 11429828
-
Corroles at work: a small macrocycle for great applications.Chem Soc Rev. 2022 Feb 21;51(4):1277-1335. doi: 10.1039/d1cs00662b. Chem Soc Rev. 2022. PMID: 35037929 Review.
Cited by
-
Phenyl derivative of iron 5,10,15-tritolylcorrole.Inorg Chem. 2014 Apr 21;53(8):4215-27. doi: 10.1021/ic5003572. Epub 2014 Apr 3. Inorg Chem. 2014. PMID: 24697623 Free PMC article.
-
One-pot synthesis of meso-alkyl substituted isocorroles: the reaction of a triarylcorrole with Grignard reagent.J Porphyr Phthalocyanines. 2010 Aug 1;14(8):752-757. doi: 10.1142/S1088424610002513. J Porphyr Phthalocyanines. 2010. PMID: 21165169 Free PMC article.
-
Open-Chain Tetrapyrroles Meet Metal Ions in the Functional Molecular Material Science.Chempluschem. 2025 Jun;90(6):e202500090. doi: 10.1002/cplu.202500090. Epub 2025 Apr 6. Chempluschem. 2025. PMID: 40126191 Free PMC article.
-
Amination reaction on copper and germanium β-nitrocorrolates.Inorg Chem. 2011 Sep 5;50(17):8281-92. doi: 10.1021/ic2008073. Epub 2011 Jul 28. Inorg Chem. 2011. PMID: 21797194 Free PMC article.
-
Corrole and nucleophilic aromatic substitution are not incompatible: a novel route to 2,3-difunctionalized copper corrolates.Org Biomol Chem. 2015 Jun 21;13(23):6611-8. doi: 10.1039/c5ob00659g. Epub 2015 May 19. Org Biomol Chem. 2015. PMID: 25986693 Free PMC article.
References
-
- Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. 1–10 Academic Press; San Diego, CA: 2000.
- Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. 11–20 Academic Press; San Diego, CA: 2003.
-
- Drain CM, Hupp JT, Suslick KS, Wasielewski MR, Chen XJ. Porphyrins Phthalocyanines. 2002;6:243–258.
-
- Sessler JL, Weghorn SJ. Expanded, Contracted and Isomeric Porphyrins. Pergamon; Oxford: 1997.
- Jasat A, Dolphin D. Chem Rev. 1997;97:2267–2340. - PubMed
- Sessler JL, Gebauer A, Vogel E. In: The Porphyrin Handbook. Kadish KM, Smith K, Guilard R, editors. Vol. 2. Academic; San Diego, CA: 2000. pp. 1–54.
- Sessler JL, Gebauer A, Weghorn SJ. In: The Porphyrin Handbook. Kadish KM, Smith K, Guilard R, editors. Vol. 2. Academic; San Diego, CA: 2000. pp. 55–124.
- Sessler JL, Seidel D. Angew Chem, Int Ed. 2003;42:5134–5175. - PubMed
- Misra R, Chandrashekar TK. Acc Chem Res. 2008;41:265–279. - PubMed
- Callot HJ, Rohrer A, Tschamber Th. New J Chem. 1995;19:155–159.
-
- Horn S, Dahms K, Senge MO. J Porphyrins Phthalocyanines. 2008;12:1053–1077.
- Jux N. Angew Chem, Int Ed. 2009;48:4284–4286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

