A synthesis of the carbon skeleton of maoecrystal V
- PMID: 19795876
- PMCID: PMC2783297
- DOI: 10.1021/ol901963v
A synthesis of the carbon skeleton of maoecrystal V
Abstract
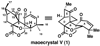
An enantioselective synthesis of the maoecrystal V (1) carbon skeleton is described. The key transformations include arylation of a 1,3-dicarbonyl compound with a triarylbismuth(V) dichloride species, oxidative dearomatization of a phenol, and a subsequent intramolecular Diels-Alder reaction.
Figures

Similar articles
-
Synthetic study toward the total synthesis of maoecrystal V.Org Lett. 2009 Nov 5;11(21):4770-3. doi: 10.1021/ol9014392. Org Lett. 2009. PMID: 19863143
-
Total synthesis of maoecrystal V.Chem Asian J. 2015 Apr;10(4):903-9. doi: 10.1002/asia.201403228. Epub 2014 Dec 12. Chem Asian J. 2015. PMID: 25504983
-
Total synthesis of (±) maoecrystal V.J Am Chem Soc. 2010 Dec 1;132(47):16745-6. doi: 10.1021/ja108907x. Epub 2010 Nov 4. J Am Chem Soc. 2010. PMID: 21049937
-
The total synthesis of Isodon diterpenes.Angew Chem Int Ed Engl. 2014 Sep 26;53(40):10588-99. doi: 10.1002/anie.201404482. Epub 2014 Aug 27. Angew Chem Int Ed Engl. 2014. PMID: 25159338 Review.
-
Enabling syntheses of diterpenoid alkaloids and related diterpenes by an oxidative dearomatization/Diels-Alder cycloaddition strategy.Nat Prod Rep. 2017 Aug 30;34(9):1044-1050. doi: 10.1039/c7np00033b. Nat Prod Rep. 2017. PMID: 28799605 Review.
Cited by
-
Directed oxidative cyclizations to C2- or C4-positions of indole: efficient construction of the bicyclo[4.3.1]decane core of welwitindolinones.Org Lett. 2011 Jun 17;13(12):3214-7. doi: 10.1021/ol201122f. Epub 2011 May 26. Org Lett. 2011. PMID: 21615098 Free PMC article.
-
Total synthesis of maoecrystal V: early-stage C-H functionalization and lactone assembly by radical cyclization.J Am Chem Soc. 2013 Oct 2;135(39):14552-5. doi: 10.1021/ja408231t. Epub 2013 Sep 23. J Am Chem Soc. 2013. PMID: 24047444 Free PMC article.
-
Enantioselective synthesis of (-)-maoecrystal V by enantiodetermining C-H functionalization.J Am Chem Soc. 2014 Dec 24;136(51):17738-49. doi: 10.1021/ja510573v. Epub 2014 Dec 11. J Am Chem Soc. 2014. PMID: 25409033 Free PMC article.
-
A synthesis of the carbocyclic core of maoecrystal V.Org Lett. 2010 Jul 2;12(13):3010-3. doi: 10.1021/ol101025r. Org Lett. 2010. PMID: 20521835 Free PMC article.
-
Oxidative Coupling of Enolates, Enol Silanes and Enamines: Methods and Natural Product Synthesis.European J Org Chem. 2012 Sep 1;2012(26):4881-4896. doi: 10.1002/ejoc.201200665. Epub 2012 Jul 16. European J Org Chem. 2012. PMID: 23471479 Free PMC article.
References
-
- Li S-H, Wang J, Niu X-M, Shen Y-H, Zhang H-J, Sun H-D, Li M-L, Tian Q-E, Lu Y, Cao P, Zheng Q-T. Org. Lett. 2004;6:4327–4330. - PubMed
-
- Sun H-D, Huang S-X, Han Q-B. Nat. Prod. Rep. 2006;23:673–698. - PubMed
-
-
During this period one of the corresponding authors of the preceding letter was employed as a postdoctoral associate (C.-C. Li) in this laboratory: Gong J, Lin G, Li C-C, Yang Z. Org. Lett. 2009, DOI: 10.1021/ol9014392. This work was reported in an NIH proposal (received 8/11/2008 and reviewed on 10/9/2008 by the BCMB-B study section) and a final report to the DFG (submitted: 7/17/2009).
-
-
- Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002;41:1668–1698. - PubMed
- Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 5. Oxford: Pergamon; 1991. pp. 513–550.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

