A synthesis of the carbon skeleton of maoecrystal V
- PMID: 19795876
- PMCID: PMC2783297
- DOI: 10.1021/ol901963v
A synthesis of the carbon skeleton of maoecrystal V
Abstract
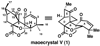
An enantioselective synthesis of the maoecrystal V (1) carbon skeleton is described. The key transformations include arylation of a 1,3-dicarbonyl compound with a triarylbismuth(V) dichloride species, oxidative dearomatization of a phenol, and a subsequent intramolecular Diels-Alder reaction.
Figures

References
-
- Li S-H, Wang J, Niu X-M, Shen Y-H, Zhang H-J, Sun H-D, Li M-L, Tian Q-E, Lu Y, Cao P, Zheng Q-T. Org. Lett. 2004;6:4327–4330. - PubMed
-
- Sun H-D, Huang S-X, Han Q-B. Nat. Prod. Rep. 2006;23:673–698. - PubMed
-
-
During this period one of the corresponding authors of the preceding letter was employed as a postdoctoral associate (C.-C. Li) in this laboratory: Gong J, Lin G, Li C-C, Yang Z. Org. Lett. 2009, DOI: 10.1021/ol9014392. This work was reported in an NIH proposal (received 8/11/2008 and reviewed on 10/9/2008 by the BCMB-B study section) and a final report to the DFG (submitted: 7/17/2009).
-
-
- Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002;41:1668–1698. - PubMed
- Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 5. Oxford: Pergamon; 1991. pp. 513–550.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

