The elastin network: its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy
- PMID: 19796069
- PMCID: PMC2796791
- DOI: 10.1111/j.1469-7580.2009.01149.x
The elastin network: its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy
Abstract
A combination of two-photon fluorescence (TPF), second harmonic generation (SHG) and coherent anti-Stokes Raman scattering (CARS) imaging has been used to investigate the elastin fibre network in healthy equine articular cartilage from the metacarpophalangeal joint. The elastin fibres were identified using their intrinsic two-photon fluorescence and immuno-staining was used to confirm the identity of these fibres. SHG was used to reveal the collagen matrix and the collagen fibre orientations were determined from their SHG polarization sensitivity, while CARS was used to clearly delineate the cell boundaries. Extensive elastin fibre networks were found in all the joint regions investigated. The elastin was found predominantly in the superficial zone (upper 50 microm) and was aligned parallel to the articular surface. Elastin was also detected in the pericellular matrix surrounding the superficial chondrocytes; however, individual fibres could not be resolved in this region. Variations in the density and organization of the fibres were observed in different regions on the joint surface.
Figures

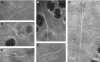




References
-
- Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. II. Der Aufbau des Gelenkknorpels in seinen Beziehungen zur Funktion. Z Zellforsch Mikrosk Anat. 1925;2:783–862.
-
- Boulesteix T, Pena AM, Pages N, et al. Micrometer scale ex vivo multiphoton imaging of unstained arterial wall structure. Cytometry A. 2006;69A:20–26. - PubMed
-
- Brama PAJ, Karssenberg D, Barneveld A, et al. Contact areas and pressure distribution on the proximal articular surface of the proximal phalanx under sagittal plane loading. Equine Vet J. 2001;33:26–32. - PubMed
-
- Brockbank KGM, MacLellan WR, Xie JS, et al. Quantitative second harmonic generation imaging of cartilage damage. Cell Tissue Banking. 2008;9:299–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

