A glycine hinge for tRNA-dependent translocation of editing substrates to prevent errors by leucyl-tRNA synthetase
- PMID: 19796639
- PMCID: PMC2807821
- DOI: 10.1016/j.febslet.2009.09.039
A glycine hinge for tRNA-dependent translocation of editing substrates to prevent errors by leucyl-tRNA synthetase
Abstract
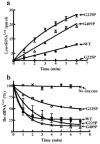
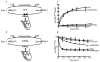
Aminoacyl-tRNA synthetases often rely on a proofreading mechanism to clear mischarging errors before they can be incorporated into newly synthesized proteins. Leucyl-tRNA synthetase (LeuRS) houses a hydrolytic editing pocket in a domain that is distinct from its aminoacylation domain. Mischarged amino acids are transiently translocated approximately 30A between active sites for editing by an unknown tRNA-dependent mechanism. A glycine within a flexible beta-strand that links the aminoacylation and editing domains of LeuRS was determined to be important to tRNA translocation. The translocation-defective mutation also demonstrated that the editing site screens both correctly and incorrectly charged tRNAs prior to product release.
Figures


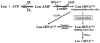
Similar articles
-
Functional segregation of a predicted "hinge" site within the beta-strand linkers of Escherichia coli leucyl-tRNA synthetase.Biochemistry. 2008 Apr 22;47(16):4808-16. doi: 10.1021/bi702494q. Epub 2008 Mar 26. Biochemistry. 2008. PMID: 18363380 Free PMC article.
-
The Yin and Yang of tRNA: proper binding of acceptor end determines the catalytic balance of editing and aminoacylation.Nucleic Acids Res. 2013 May 1;41(10):5513-23. doi: 10.1093/nar/gkt252. Epub 2013 Apr 12. Nucleic Acids Res. 2013. PMID: 23585282 Free PMC article.
-
Modulation of Aminoacylation and Editing Properties of Leucyl-tRNA Synthetase by a Conserved Structural Module.J Biol Chem. 2015 May 8;290(19):12256-67. doi: 10.1074/jbc.M115.639492. Epub 2015 Mar 27. J Biol Chem. 2015. PMID: 25817995 Free PMC article.
-
Trans-editing by aminoacyl-tRNA synthetase-like editing domains.Enzymes. 2020;48:69-115. doi: 10.1016/bs.enz.2020.07.002. Epub 2020 Sep 8. Enzymes. 2020. PMID: 33837712 Review.
-
Energy cost of translational proofreading in vivo. The aminoacylation of transfer RNA in Escherichia coli.Ann N Y Acad Sci. 1994 Nov 30;745:4-20. doi: 10.1111/j.1749-6632.1994.tb44360.x. Ann N Y Acad Sci. 1994. PMID: 7530434 Review.
Cited by
-
tRNA-dependent pre-transfer editing by prokaryotic leucyl-tRNA synthetase.J Biol Chem. 2010 Jan 29;285(5):3235-44. doi: 10.1074/jbc.M109.060616. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940155 Free PMC article.
-
tRNA synthetase: tRNA aminoacylation and beyond.Wiley Interdiscip Rev RNA. 2014 Jul-Aug;5(4):461-80. doi: 10.1002/wrna.1224. Epub 2014 Apr 4. Wiley Interdiscip Rev RNA. 2014. PMID: 24706556 Free PMC article. Review.
References
-
- Mascarenhas AP, An S, Rosen AE, Martinis SA, Musier-Forsyth K. Fidelity Mechanisms of the Aminoacyl-tRNA Synthetases. In: RajBhandary UL, Köhrer C, editors. Protein Engineering. Springer Verlag; 2007. pp. 153–200.
-
- Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–5. - PubMed
-
- Nangle LA, Motta CM, Schimmel P. Global effects of mistranslation from an editing defect in mammalian cells. Chem Biol. 2006;13:1091–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

