A glycine hinge for tRNA-dependent translocation of editing substrates to prevent errors by leucyl-tRNA synthetase
- PMID: 19796639
- PMCID: PMC2807821
- DOI: 10.1016/j.febslet.2009.09.039
A glycine hinge for tRNA-dependent translocation of editing substrates to prevent errors by leucyl-tRNA synthetase
Abstract
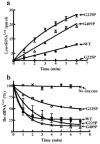
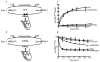
Aminoacyl-tRNA synthetases often rely on a proofreading mechanism to clear mischarging errors before they can be incorporated into newly synthesized proteins. Leucyl-tRNA synthetase (LeuRS) houses a hydrolytic editing pocket in a domain that is distinct from its aminoacylation domain. Mischarged amino acids are transiently translocated approximately 30A between active sites for editing by an unknown tRNA-dependent mechanism. A glycine within a flexible beta-strand that links the aminoacylation and editing domains of LeuRS was determined to be important to tRNA translocation. The translocation-defective mutation also demonstrated that the editing site screens both correctly and incorrectly charged tRNAs prior to product release.
Figures


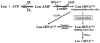
References
-
- Mascarenhas AP, An S, Rosen AE, Martinis SA, Musier-Forsyth K. Fidelity Mechanisms of the Aminoacyl-tRNA Synthetases. In: RajBhandary UL, Köhrer C, editors. Protein Engineering. Springer Verlag; 2007. pp. 153–200.
-
- Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–5. - PubMed
-
- Nangle LA, Motta CM, Schimmel P. Global effects of mistranslation from an editing defect in mammalian cells. Chem Biol. 2006;13:1091–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

