Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target
- PMID: 19796750
- PMCID: PMC2935850
- DOI: 10.1016/S1470-2045(09)70229-3
Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target
Abstract
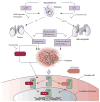
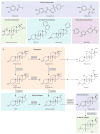
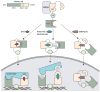
Activation of the androgen receptor is crucial for prostate cancer growth at all points of the illness. Current therapies targeting the androgen receptor, including androgen-depletion approaches and anti-androgens, do not completely inhibit the receptor activity. Prostate cancer cells develop resistance to castration by acquiring changes that include androgen-receptor overexpression and overexpression of enzymes involved in androgen biosynthesis, which result in reactivation of the receptor. Based on an understanding of these resistance mechanisms and androgen biosynthesis pathways, new anti-androgens and androgen-depleting agents have been developed. Notably, promising activity has been shown in early phase trials by MDV3100, a new anti-androgen designed for activity in prostate cancer model systems with overexpressed androgen receptor, and by abiraterone acetate, a CYP17A inhibitor that blocks steroid biosynthesis in the adrenal gland and possibly within the tumour. Both agents are undergoing phase 3 testing. Here, we review the basic science and clinical development of these and other agents.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
Comment in
-
Words of wisdom. Re: anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target.Eur Urol. 2010 Feb;57(2):356-7. doi: 10.1016/j.eururo.2009.11.019. Eur Urol. 2010. PMID: 20116769 No abstract available.
References
-
- Griffin JE. Androgen resistance--the clinical and molecular spectrum. N Engl J Med. 1992 Feb 27;326(9):611–8. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1513–20. - PubMed
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502–12. - PubMed
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001 Oct;1(1):34–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

