Mapping gray matter development: implications for typical development and vulnerability to psychopathology
- PMID: 19796863
- PMCID: PMC2815268
- DOI: 10.1016/j.bandc.2009.08.009
Mapping gray matter development: implications for typical development and vulnerability to psychopathology
Abstract
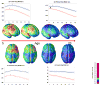
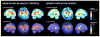
Recent studies with brain magnetic resonance imaging (MRI) have scanned large numbers of children and adolescents repeatedly over time, as their brains develop, tracking volumetric changes in gray and white matter in remarkable detail. Focusing on gray matter changes specifically, here we explain how earlier studies using lobar volumes of specific anatomical regions showed how different lobes of the brain matured at different rates. With the advent of more sophisticated brain mapping methods, it became possible to chart the dynamic trajectory of cortical maturation using detailed 3D and 4D (dynamic) models, showing spreading waves of changes evolving through the cortex. This led to a variety of time-lapse films revealing characteristic deviations from normal development in schizophrenia, bipolar illness, and even in siblings at genetic risk for these disorders. We describe how these methods have helped clarify how cortical development relates to cognitive performance, functional recovery or decline in illness, and ongoing myelination processes. These time-lapse maps have also been used to study effects of genotype and medication on cortical maturation, presenting a powerful framework to study factors that influence the developing brain.
Published by Elsevier Inc.
Figures




Similar articles
-
Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia.Neuroimage. 2004;23 Suppl 1:S2-18. doi: 10.1016/j.neuroimage.2004.07.071. Neuroimage. 2004. PMID: 15501091 Review.
-
Gray matter abnormalities as brain structural vulnerability factors for bipolar disorder: A review of neuroimaging studies of individuals at high genetic risk for bipolar disorder.Aust N Z J Psychiatry. 2013 Dec;47(12):1124-35. doi: 10.1177/0004867413496482. Epub 2013 Jul 17. Aust N Z J Psychiatry. 2013. PMID: 23864160 Review.
-
Subcomponents of brain T2* relaxation in schizophrenia, bipolar disorder and siblings: A Gradient Echo Plural Contrast Imaging (GEPCI) study.Schizophr Res. 2015 Dec;169(1-3):36-45. doi: 10.1016/j.schres.2015.10.004. Epub 2015 Oct 23. Schizophr Res. 2015. PMID: 26603058 Free PMC article.
-
Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder.Biol Psychiatry. 2005 Nov 1;58(9):713-23. doi: 10.1016/j.biopsych.2005.04.033. Epub 2005 Jul 5. Biol Psychiatry. 2005. PMID: 15993858
-
Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder.Biol Psychiatry. 2010 Jun 1;67(11):1097-105. doi: 10.1016/j.biopsych.2010.01.020. Epub 2010 Mar 29. Biol Psychiatry. 2010. PMID: 20303066
Cited by
-
Profiling intra- and inter-individual differences in brain development across early adolescence.Neuroimage. 2023 Oct 1;279:120287. doi: 10.1016/j.neuroimage.2023.120287. Epub 2023 Aug 1. Neuroimage. 2023. PMID: 37536527 Free PMC article.
-
Investigation of brain structure in the 1-month infant.Brain Struct Funct. 2018 May;223(4):1953-1970. doi: 10.1007/s00429-017-1600-2. Epub 2018 Jan 5. Brain Struct Funct. 2018. PMID: 29305647 Free PMC article.
-
Neuroanatomical Correlates of Perceived Stress Controllability in Adolescents and Emerging Adults.Cogn Affect Behav Neurosci. 2022 Aug;22(4):655-671. doi: 10.3758/s13415-022-00985-2. Epub 2022 Jan 28. Cogn Affect Behav Neurosci. 2022. PMID: 35091987 Free PMC article.
-
Greater maltreatment severity is associated with smaller brain volume with implication for intellectual ability in young children.Neurobiol Stress. 2023 Sep 23;27:100576. doi: 10.1016/j.ynstr.2023.100576. eCollection 2023 Nov. Neurobiol Stress. 2023. PMID: 37810429 Free PMC article.
-
The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence.Brain Struct Funct. 2018 Mar;223(2):669-685. doi: 10.1007/s00429-017-1509-9. Epub 2017 Sep 14. Brain Struct Funct. 2018. PMID: 28913599 Free PMC article.
References
-
- Asarnow RF, Asamen J, Granholm E, Sherman T, Watkins JM, Williams ME. Cognitive/neuropsychological studies of children with a schizophrenic disorder. Schizophr Bull. 1994;20(4):647–669. - PubMed
-
- Asarnow RF, Nuechterlein KH, Fogelson D, Subotnik KL, Payne DA, Russell AT, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58(6):581–588. - PubMed
-
- Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2(2):79–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

