A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes
- PMID: 19796917
- PMCID: PMC2822066
- DOI: 10.1016/j.jaut.2009.08.012
A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes
Abstract
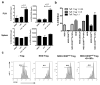
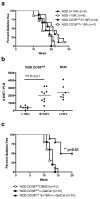
The ectoenzyme ADP-ribosyltransferase 2.2 (ART2.2) can apoptotically delete various T-cell subsets. Depending on the involved apoptotic T-cell subset, enhanced ART2.2 activity could result in immunosuppression or autoimmunity. Diminished activity of the CD38 ectoenzyme that normally represents a counter-regulatory competitor for the NAD substrate represents one mechanism enhancing ART2.2 activity. Hence, it would be desirable to develop an agent that efficiently blocks ART2.2 activity in vivo. While the llama derived recombinant s+16 single domain antibody overcame the difficulty of specifically targeting the ART2.2 catalytic site potential therapeutic use of this reagent is limited due to short in vivo persistence. Thus, we tested if a modified version of s+16 incorporating the murine IgG1 Fc tail (s+16Fc) mediated long-term efficient in vivo suppression of ART2.2. We reasoned an ideal model to test the s+16Fc reagent were NOD mice in which genetic ablation of CD38 results in an ART2.2 mediated reduction in already sub-normal numbers of immunoregulatory natural killer T-(NKT) cells to a level that no longer allows them when activated by the super-agonist alpha-galactosylceramide (alpha-GalCer) to elicit effects inhibiting autoimmune type 1 diabetes (T1D) development. Treatment with s+16Fc efficiently mediated long-term in vivo inhibition of ART2.2 activity in NOD.CD38(null) mice, restoring their iNKT cell numbers to levels that upon alpha-GalCer activation were capable of inhibiting T1D development.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Testing the role of P2X7 receptors in the development of type 1 diabetes in nonobese diabetic mice.J Immunol. 2011 Apr 1;186(7):4278-84. doi: 10.4049/jimmunol.1003733. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357538 Free PMC article.
-
Targeted disruption of CD38 accelerates autoimmune diabetes in NOD/Lt mice by enhancing autoimmunity in an ADP-ribosyltransferase 2-dependent fashion.J Immunol. 2006 Apr 15;176(8):4590-9. doi: 10.4049/jimmunol.176.8.4590. J Immunol. 2006. PMID: 16585549
-
Changing patterns of cell surface mono (ADP-ribosyl) transferase antigen ART2.2 on resting versus cytopathically-activated T cells in NOD/Lt mice.Diabetologia. 2001 Jul;44(7):848-58. doi: 10.1007/s001250100559. Diabetologia. 2001. PMID: 11508269
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses.J Biomed Sci. 2017 Mar 23;24(1):22. doi: 10.1186/s12929-017-0325-0. J Biomed Sci. 2017. PMID: 28335781 Free PMC article. Review.
-
Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities.Tissue Antigens. 2009 Jun;73(6):535-45. doi: 10.1111/j.1399-0039.2009.01256.x. Epub 2009 Apr 8. Tissue Antigens. 2009. PMID: 19392798 Review.
Cited by
-
Administration of an AAV vector coding for a P2X7-blocking nanobody-based biologic ameliorates colitis in mice.J Nanobiotechnology. 2024 Jan 11;22(1):27. doi: 10.1186/s12951-023-02285-4. J Nanobiotechnology. 2024. PMID: 38212782 Free PMC article.
-
Tumour immune escape via P2X7 receptor signalling.Front Immunol. 2023 Oct 30;14:1287310. doi: 10.3389/fimmu.2023.1287310. eCollection 2023. Front Immunol. 2023. PMID: 38022596 Free PMC article. Review.
-
P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption.World J Gastroenterol. 2022 Sep 28;28(36):5265-5279. doi: 10.3748/wjg.v28.i36.5265. World J Gastroenterol. 2022. PMID: 36185635 Free PMC article. Review.
-
Interferon-γ Limits Diabetogenic CD8+ T-Cell Effector Responses in Type 1 Diabetes.Diabetes. 2017 Mar;66(3):710-721. doi: 10.2337/db16-0846. Epub 2016 Dec 5. Diabetes. 2017. PMID: 27920091 Free PMC article.
-
Testing the role of P2X7 receptors in the development of type 1 diabetes in nonobese diabetic mice.J Immunol. 2011 Apr 1;186(7):4278-84. doi: 10.4049/jimmunol.1003733. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357538 Free PMC article.
References
-
- Okamoto S, Azhipa O, Yu Y, Russo E, Dennert G. Expression of ADP-ribosyltransferase on normal T lymphocytes and effects of nicotinamide adenine dinucleotide on their function. J Immunol. 1998;160:4190–8. - PubMed
-
- Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–82. - PubMed
-
- Krebs C, Adriouch S, Braasch F, Koestner W, Leiter EH, Seman M, Lund FE, Oppenheimer N, Haag F, Koch-Nolte F. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J Immunol. 2005;174:3298–305. - PubMed
-
- Chen J, Chen YG, Reifsnyder PC, Schott WH, Lee CH, Osborne M, Scheuplein F, Haag F, Koch-Nolte F, Serreze DV, et al. Targeted disruption of CD38 accelerates autoimmune diabetes in NOD/Lt mice by enhancing autoimmunity in an ADP-ribosyltransferase 2-dependent fashion. J Immunol. 2006;176:4590–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

