Pharmacogenetic analysis reveals a post-developmental role for Rac GTPases in Caenorhabditis elegans GABAergic neurotransmission
- PMID: 19797046
- PMCID: PMC2784297
- DOI: 10.1534/genetics.109.106880
Pharmacogenetic analysis reveals a post-developmental role for Rac GTPases in Caenorhabditis elegans GABAergic neurotransmission
Abstract
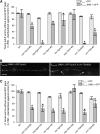
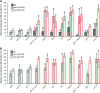
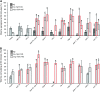
The nerve-cell cytoskeleton is essential for the regulation of intrinsic neuronal activity. For example, neuronal migration defects are associated with microtubule regulators, such as LIS1 and dynein, as well as with actin regulators, including Rac GTPases and integrins, and have been thought to underlie epileptic seizures in patients with cortical malformations. However, it is plausible that post-developmental functions of specific cytoskeletal regulators contribute to the more transient nature of aberrant neuronal activity and could be masked by developmental anomalies. Accordingly, our previous results have illuminated functional roles, distinct from developmental contributions, for Caenorhabditis elegans orthologs of LIS1 and dynein in GABAergic synaptic vesicle transport. Here, we report that C. elegans with function-altering mutations in canonical Rac GTPase-signaling-pathway members demonstrated a robust behavioral response to a GABA(A) receptor antagonist, pentylenetetrazole. Rac mutants also exhibited hypersensitivity to an acetylcholinesterase inhibitor, aldicarb, uncovering deficiencies in inhibitory neurotransmission. RNA interference targeting Rac hypomorphs revealed synergistic interactions between the dynein motor complex and some, but not all, members of Rac-signaling pathways. These genetic interactions are consistent with putative Rac-dependent regulation of actin and microtubule networks and suggest that some cytoskeletal regulators cooperate to uniquely govern neuronal synchrony through dynein-mediated GABAergic vesicle transport in C. elegans.
Figures





Similar articles
-
Paradigms for pharmacological characterization of C. elegans synaptic transmission mutants.J Vis Exp. 2008 Aug 18;(18):837. doi: 10.3791/837. J Vis Exp. 2008. PMID: 19066504 Free PMC article.
-
Epileptic-like convulsions associated with LIS-1 in the cytoskeletal control of neurotransmitter signaling in Caenorhabditis elegans.Hum Mol Genet. 2004 Sep 15;13(18):2043-59. doi: 10.1093/hmg/ddh209. Epub 2004 Jul 14. Hum Mol Genet. 2004. PMID: 15254012
-
Genetic interactions among cortical malformation genes that influence susceptibility to convulsions in C. elegans.Brain Res. 2006 Nov 20;1120(1):23-34. doi: 10.1016/j.brainres.2006.08.067. Epub 2006 Sep 22. Brain Res. 2006. PMID: 16996038
-
Control of developmental networks by Rac/Rho small GTPases: How cytoskeletal changes during embryogenesis are orchestrated.Bioessays. 2016 Dec;38(12):1246-1254. doi: 10.1002/bies.201600165. Epub 2016 Oct 28. Bioessays. 2016. PMID: 27790724 Free PMC article. Review.
-
Roles of Rac1 and Rac3 GTPases during the development of cortical and hippocampal GABAergic interneurons.Front Cell Neurosci. 2014 Sep 25;8:307. doi: 10.3389/fncel.2014.00307. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25309333 Free PMC article. Review.
Cited by
-
NCEH-1 modulates cholesterol metabolism and protects against α-synuclein toxicity in a C. elegans model of Parkinson's disease.Hum Mol Genet. 2017 Oct 1;26(19):3823-3836. doi: 10.1093/hmg/ddx269. Hum Mol Genet. 2017. PMID: 28934392 Free PMC article.
-
A Behavioral Survey of the Effects of Kavalactones on Caenorhabditis elegans Neuromuscular Transmission.J Exp Neurosci. 2017 Jun 5;11:1179069517705384. doi: 10.1177/1179069517705384. eCollection 2017. J Exp Neurosci. 2017. PMID: 28615969 Free PMC article.
-
Integrins Have Cell-Type-Specific Roles in the Development of Motor Neuron Connectivity.J Dev Biol. 2019 Aug 27;7(3):17. doi: 10.3390/jdb7030017. J Dev Biol. 2019. PMID: 31461926 Free PMC article.
-
VAV-1 acts in a single interneuron to inhibit motor circuit activity in Caenorhabditis elegans.Nat Commun. 2014 Nov 21;5:5579. doi: 10.1038/ncomms6579. Nat Commun. 2014. PMID: 25412913 Free PMC article.
-
Using C. elegans to decipher the cellular and molecular mechanisms underlying neurodevelopmental disorders.Mol Neurobiol. 2013 Dec;48(3):465-89. doi: 10.1007/s12035-013-8434-6. Epub 2013 Mar 14. Mol Neurobiol. 2013. PMID: 23494747 Review.
References
-
- Arion, D., M. Sabatini, T. Unger, J. Pastor, L. Alonso-Nanclares et al., 2006. Correlation of transcriptome profile with electrical activity in temporal lobe epilepsy. Neurobiol. Dis. 22 374–387. - PubMed
-
- Baraban, S. C., M. R. Taylor, P. A. Castro and H. Baier, 2005. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131 759–768. - PubMed
-
- Baum, P. D., and G. Garriga, 1997. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19 51–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

