Myocardin-dependent activation of the CArG box-rich smooth muscle gamma-actin gene: preferential utilization of a single CArG element through functional association with the NKX3.1 homeodomain protein
- PMID: 19797053
- PMCID: PMC2781672
- DOI: 10.1074/jbc.M109.033910
Myocardin-dependent activation of the CArG box-rich smooth muscle gamma-actin gene: preferential utilization of a single CArG element through functional association with the NKX3.1 homeodomain protein
Abstract
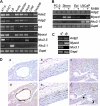
Serum response factor (SRF) is a ubiquitously expressed transcription factor that binds a 10-bp element known as the CArG box, located in the proximal regulatory region of hundreds of target genes. SRF activates target genes in a cell- and context-dependent manner by assembling unique combinations of cofactors over CArG elements. One particularly strong SRF cofactor, myocardin (MYOCD), acts as a component of a molecular switch for smooth muscle cell (SMC) differentiation by activating cytoskeletal and contractile genes harboring SRF-binding CArG elements. Here we report that the human ACTG2 promoter, containing four conserved CArG elements, displays SMC-specific basal activity and is highly induced in the presence of MYOCD. Stable transfection of a non-SMC cell type with Myocd elicits elevations in endogenous Actg2 mRNA. Gel shift and luciferase assays reveal a strong bias for MYOCD-dependent transactivation through CArG2 of the human ACTG2 promoter. Substitution of CArG2 with other CArGs, including a consensus CArG element, fails to reconstitute full MYOCD-dependent ACTG2 promoter stimulation. Mutation of an adjacent binding site for NKX3.1 reduces MYOCD-dependent transactivation of the ACTG2 promoter. Co-immunoprecipitation, glutathione S-transferase pulldown, and luciferase assays show a physical and functional association between MYOCD and NKX3.1; no such functional relationship is evident with the related NKX2.5 transcription factor despite its interaction with MYOCD. These results demonstrate the ability of MYOCD to discriminate among several juxtaposed CArG elements, presumably through its novel partnership with NKX3.1, to optimally transactivate the human ACTG2 promoter.
Figures




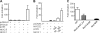

Similar articles
-
Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo.Circ Res. 2005 Nov 11;97(10):983-91. doi: 10.1161/01.RES.0000190604.90049.71. Epub 2005 Oct 13. Circ Res. 2005. PMID: 16224064
-
The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin.J Biol Chem. 2009 Nov 27;284(48):33671-82. doi: 10.1074/jbc.M109.050419. Epub 2009 Oct 1. J Biol Chem. 2009. PMID: 19801679 Free PMC article.
-
Leiomodin 1, a new serum response factor-dependent target gene expressed preferentially in differentiated smooth muscle cells.J Biol Chem. 2012 Jan 20;287(4):2459-67. doi: 10.1074/jbc.M111.302224. Epub 2011 Dec 7. J Biol Chem. 2012. PMID: 22157009 Free PMC article.
-
Serum response factor: toggling between disparate programs of gene expression.J Mol Cell Cardiol. 2003 Jun;35(6):577-93. doi: 10.1016/s0022-2828(03)00110-x. J Mol Cell Cardiol. 2003. PMID: 12788374 Review.
-
Emerging roles of the myocardin family of proteins in lipid and glucose metabolism.J Physiol. 2016 Sep 1;594(17):4741-52. doi: 10.1113/JP271913. Epub 2016 May 10. J Physiol. 2016. PMID: 27060572 Free PMC article. Review.
Cited by
-
In search of novel targets for heart disease: myocardin and myocardin-related transcriptional cofactors.Biochem Res Int. 2012;2012:973723. doi: 10.1155/2012/973723. Epub 2012 May 17. Biochem Res Int. 2012. PMID: 22666593 Free PMC article.
-
Myocardin and Stat3 act synergistically to inhibit cardiomyocyte apoptosis.Oncotarget. 2017 Aug 24;8(59):99612-99623. doi: 10.18632/oncotarget.20450. eCollection 2017 Nov 21. Oncotarget. 2017. PMID: 29245928 Free PMC article.
-
Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis.J Biomed Res. 2014 Jan;28(1):25-39. doi: 10.7555/JBR.27.20130064. Epub 2013 Sep 20. J Biomed Res. 2014. PMID: 24474961 Free PMC article. Review.
-
The benign nature and rare occurrence of cardiac myxoma as a possible consequence of the limited cardiac proliferative/ regenerative potential: a systematic review.BMC Cancer. 2023 Dec 18;23(1):1245. doi: 10.1186/s12885-023-11723-3. BMC Cancer. 2023. PMID: 38110859 Free PMC article.
-
TonEBP/NFAT5 regulates ACTBL2 expression in biomechanically activated vascular smooth muscle cells.Front Physiol. 2014 Dec 3;5:467. doi: 10.3389/fphys.2014.00467. eCollection 2014. Front Physiol. 2014. PMID: 25520667 Free PMC article.
References
-
- Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Physiol. Rev. 84, 767–801 - PubMed
-
- Miano J. M. (2003) J. Mol. Cell. Cardiol. 35, 577–593 - PubMed
-
- Wang D., Chang P. S., Wang Z., Sutherland L., Richardson J. A., Small E., Krieg P. A., Olson E. N. (2001) Cell 105, 851–862 - PubMed
-
- Chen J., Kitchen C. M., Streb J. W., Miano J. M. (2002) J. Mol. Cell. Cardiol. 34, 1345–1356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

