OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis
- PMID: 19797074
- PMCID: PMC2786482
- DOI: 10.1128/IAI.00670-09
OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis
Abstract
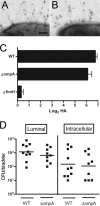
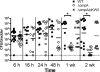
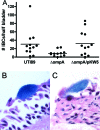
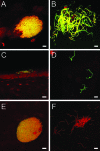
Type 1 pilus directs bladder epithelial binding and invasion by uropathogenic Escherichia coli (UPEC) in the initial stage of cystitis, but the bacterial determinants of postinvasion events in the pathogenesis of cystitis are largely undetermined. We show here that the UPEC outer membrane protein A (OmpA), a monomeric, major, integral protein component of the bacterial outer membrane, functions as a critical determinant of intracellular virulence for UPEC, promoting persistent infection within bladder epithelium. Using a murine urinary tract infection (UTI) model, we demonstrate that whereas deletion of the UPEC ompA gene did not disrupt initial epithelial binding and invasion by UPEC, it did preclude completion of the intracellular bacterial community (IBC) pathway, accompanied by diminishing bacterial loads in the bladder. This defect in epithelial persistence of the ompA mutant was enhanced in competitive infections with wild-type UPEC. Microscopic examinations revealed that the ompA mutant formed significantly fewer IBCs, and those that were initiated were unable to progress past the early stages of maturation. These defects could be corrected by complementation of ompA. In addition, expression of ompA during wild-type UTI was sharply increased at time points correlated with IBC development and the arrival of host immune effector cells. Our findings establish OmpA as a key UPEC virulence factor that functions after epithelial invasion to facilitate IBC maturation and chronic bacterial persistence.
Figures


Similar articles
-
Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection.FEMS Microbiol Rev. 2012 May;36(3):616-48. doi: 10.1111/j.1574-6976.2012.00339.x. FEMS Microbiol Rev. 2012. PMID: 22404313 Free PMC article. Review.
-
Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection.Infect Immun. 2011 Oct;79(10):4250-9. doi: 10.1128/IAI.05339-11. Epub 2011 Aug 1. Infect Immun. 2011. PMID: 21807904 Free PMC article.
-
Metabolic Requirements of Escherichia coli in Intracellular Bacterial Communities during Urinary Tract Infection Pathogenesis.mBio. 2016 Apr 12;7(2):e00104-16. doi: 10.1128/mBio.00104-16. mBio. 2016. PMID: 27073089 Free PMC article.
-
Maturation of intracellular Escherichia coli communities requires SurA.Infect Immun. 2006 Aug;74(8):4793-800. doi: 10.1128/IAI.00355-06. Infect Immun. 2006. PMID: 16861667 Free PMC article.
-
Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli.Eur J Clin Invest. 2008 Oct;38 Suppl 2:2-11. doi: 10.1111/j.1365-2362.2008.01986.x. Epub 2008 Jul 8. Eur J Clin Invest. 2008. PMID: 18616559 Review.
Cited by
-
AOA-2 Derivatives as Outer Membrane Protein A Inhibitors for Treatment of Gram-Negative Bacilli Infections.Front Microbiol. 2021 Feb 12;12:634323. doi: 10.3389/fmicb.2021.634323. eCollection 2021. Front Microbiol. 2021. PMID: 33643267 Free PMC article.
-
Proteome Exploration of Legionella pneumophila To Identify Novel Therapeutics: a Hierarchical Subtractive Genomics and Reverse Vaccinology Approach.Microbiol Spectr. 2022 Aug 31;10(4):e0037322. doi: 10.1128/spectrum.00373-22. Epub 2022 Jul 12. Microbiol Spectr. 2022. PMID: 35863001 Free PMC article.
-
Characterization and comparative analysis of the Escherichia marmotae M-12 isolate from bank vole (Myodes glareolus).Sci Rep. 2023 Aug 25;13(1):13949. doi: 10.1038/s41598-023-41223-0. Sci Rep. 2023. PMID: 37626115 Free PMC article.
-
Biological Functions of Prokaryotic Amyloids in Interspecies Interactions: Facts and Assumptions.Int J Mol Sci. 2020 Sep 30;21(19):7240. doi: 10.3390/ijms21197240. Int J Mol Sci. 2020. PMID: 33008049 Free PMC article. Review.
-
Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection.FEMS Microbiol Rev. 2012 May;36(3):616-48. doi: 10.1111/j.1574-6976.2012.00339.x. FEMS Microbiol Rev. 2012. PMID: 22404313 Free PMC article. Review.
References
-
- Anderson, G. G., K. W. Dodson, T. M. Hooton, and S. J. Hultgren. 2004. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 12:424-430. - PubMed
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Arora, A., F. Abildgaard, J. H. Bushweller, and L. K. Tamm. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat. Struct. Biol. 8:334-338. - PubMed
-
- Arora, A., D. Rinehart, G. Szabo, and L. K. Tamm. 2000. Refolded outer membrane protein A of Escherichia coli forms ion channels with two conductance states in planar lipid bilayers. J. Biol. Chem. 275:1594-1600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

