Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes
- PMID: 19797076
- PMCID: PMC2753151
- DOI: 10.1083/jcb.200907014
Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes
Abstract
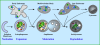
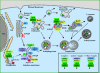
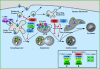
Autophagy or "self-eating" is a highly conserved pathway that enables cells to degrade pieces of themselves in autolysosomes to enable their survival in times of stress, including nutrient deprivation. The formation of these degradative compartments requires cytosolic proteins, some of which are autophagy specific, as well as intracellular organelles, such as the ER and Golgi, and the endosome-lysosome system. Here we discuss the cross talk between autophagy and intracellular compartments, highlighting recent exciting data about the role and regulation of the Vps34 class III phosphatidylinositol (PI) 3-kinase in autophagy.
Figures
References
-
- Audhya A., Desai A., Oegema K. 2007. A role for Rab5 in structuring the endoplasmic reticulum.J. Cell Biol. 178:43–56 doi:10.1083/jcb.200701139 - DOI - PMC - PubMed
-
- Axe E.L., Walker S.A., Manifava M., Chandra P., Roderick H.L., Habermann A., Griffiths G., Ktistakis N.T. 2008. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum.J. Cell Biol. 182:685–701 doi:10.1083/jcb.200803137 - DOI - PMC - PubMed
-
- Babst M., Wendland B., Estepa E.J., Emr S.D. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function.EMBO J. 17:2982–2993 doi:10.1093/emboj/17.11.2982 - DOI - PMC - PubMed
-
- Backer J.M. 2008. The regulation and function of Class III PI3Ks: novel roles for Vps34.Biochem. J. 410:1–17 doi:10.1042/BJ20071427 - DOI - PubMed
-
- Burda P., Padilla S.M., Sarkar S., Emr S.D. 2002. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase.J. Cell Sci. 115:3889–3900 doi:10.1242/jcs.00090 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

