Yvh1 is required for a late maturation step in the 60S biogenesis pathway
- PMID: 19797079
- PMCID: PMC2753168
- DOI: 10.1083/jcb.200904111
Yvh1 is required for a late maturation step in the 60S biogenesis pathway
Abstract
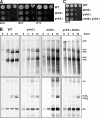
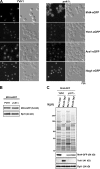
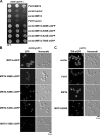
Before entering translation, preribosomal particles undergo sequential late maturation steps. In the case of pre-60S particles, these steps involve the release of shuttling maturation factors and transport receptors. In this study, we report a new maturation step in the 60S biogenesis pathway in budding yeast. We show that efficient release of the nucleolar/nuclear ribosomal-like protein Mrt4 (homologous to the acidic ribosomal P-protein Rpp0) from pre-60S particles requires the highly conserved protein Yvh1, which associates only with late pre-60S particles. Cell biological and biochemical analyses reveal that Mrt4 fails to dissociate from late pre-60S particles in yvh1Delta cells, inducing a delay in nuclear pre-ribosomal RNA processing and a pre-60S export defect in yvh1Delta cells. Moreover, we have isolated gain of function alleles of Mrt4 that specifically bypass the requirement for Yvh1 and rescue all yvh1Delta-associated phenotypes. Together, our data suggest that Yvh1-mediated release of Mrt4 precedes cytoplasmic loading of Rpp0 on pre-60S particles and is an obligatory late step toward construction of translation-competent 60S subunits.
Figures


Similar articles
-
Ribosome stalk assembly requires the dual-specificity phosphatase Yvh1 for the exchange of Mrt4 with P0.J Cell Biol. 2009 Sep 21;186(6):849-62. doi: 10.1083/jcb.200904110. J Cell Biol. 2009. PMID: 19797078 Free PMC article.
-
Genetic interactions of ribosome maturation factors Yvh1 and Mrt4 influence mRNA decay, glycogen accumulation, and the expression of early meiotic genes in Saccharomyces cerevisiae.J Biochem. 2011 Jul;150(1):103-11. doi: 10.1093/jb/mvr040. Epub 2011 Apr 6. J Biochem. 2011. PMID: 21474464
-
The conserved Bud20 zinc finger protein is a new component of the ribosomal 60S subunit export machinery.Mol Cell Biol. 2012 Dec;32(24):4898-912. doi: 10.1128/MCB.00910-12. Epub 2012 Oct 8. Mol Cell Biol. 2012. PMID: 23045392 Free PMC article.
-
Nuclear export and cytoplasmic maturation of ribosomal subunits.FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review.
-
Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast.Biochem J. 2017 Jan 15;474(2):195-214. doi: 10.1042/BCJ20160516. Biochem J. 2017. PMID: 28062837 Free PMC article. Review.
Cited by
-
Targeted proteomics reveals compositional dynamics of 60S pre-ribosomes after nuclear export.Mol Syst Biol. 2012;8:628. doi: 10.1038/msb.2012.63. Mol Syst Biol. 2012. PMID: 23212245 Free PMC article.
-
P1 and P2 protein heterodimer binding to the P0 protein of Saccharomyces cerevisiae is relatively non-specific and a source of ribosomal heterogeneity.Nucleic Acids Res. 2012 May;40(10):4520-9. doi: 10.1093/nar/gks036. Epub 2012 Jan 24. Nucleic Acids Res. 2012. PMID: 22275522 Free PMC article.
-
The ribosome assembly factor Nop53 has a structural role in the formation of nuclear pre-60S intermediates, affecting late maturation events.Nucleic Acids Res. 2021 Jul 9;49(12):7053-7074. doi: 10.1093/nar/gkab494. Nucleic Acids Res. 2021. PMID: 34125911 Free PMC article.
-
Carboxy terminal modifications of the P0 protein reveal alternative mechanisms of nuclear ribosomal stalk assembly.Nucleic Acids Res. 2013 Oct;41(18):8628-36. doi: 10.1093/nar/gkt637. Epub 2013 Jul 23. Nucleic Acids Res. 2013. PMID: 23880660 Free PMC article.
-
Non-FG mediated transport of the large pre-ribosomal subunit through the nuclear pore complex by the mRNA export factor Gle2.Nucleic Acids Res. 2013 Sep;41(17):8266-79. doi: 10.1093/nar/gkt675. Epub 2013 Jul 31. Nucleic Acids Res. 2013. PMID: 23907389 Free PMC article.
References
-
- Aoki N., Aoyama K., Nagata M., Matsuda T. 2001. A growing family of dual specificity phosphatases with low molecular masses.J. Biochem. 130:133–140 - PubMed
-
- Bassler J., Grandi P., Gadal O., Lessmann T., Petfalski E., Tollervey D., Lechner J., Hurt E. 2001. Identification of a 60S preribosomal particle that is closely linked to nuclear export.Mol. Cell. 8:517–529 doi:10.1016/S1097-2765(01)00342-2 - DOI - PubMed
-
- Bradatsch B., Katahira J., Kowalinski E., Bange G., Yao W., Sekimoto T., Baumgärtel V., Boese G., Bassler J., Wild K., et al. 2007. Arx1 functions as an unorthodox nuclear export receptor for the 60S preribosomal subunit.Mol. Cell. 27:767–779 doi:10.1016/j.molcel.2007.06.034 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

