UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans
- PMID: 19797081
- PMCID: PMC2753160
- DOI: 10.1083/jcb.200902096
UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans
Abstract
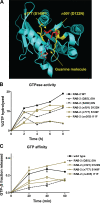
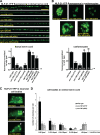
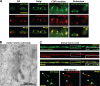
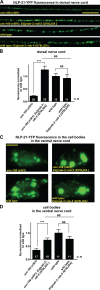
Small guanosine triphosphatases of the Rab family regulate intracellular vesicular trafficking. Rab2 is highly expressed in the nervous system, yet its function in neurons is unknown. In Caenorhabditis elegans, unc-108/rab-2 mutants have been isolated based on their locomotory defects. We show that the locomotion defects of rab-2 mutants are not caused by defects in synaptic vesicle release but by defects in dense core vesicle (DCV) signaling. DCVs in rab-2 mutants are often enlarged and heterogeneous in size; however, their number and distribution are not affected. This implicates Rab2 in the biogenesis of DCVs at the Golgi complex. We demonstrate that Rab2 is required to prevent DCV cargo from inappropriately entering late endosomal compartments during DCV maturation. Finally, we show that RIC-19, the C. elegans orthologue of the human diabetes autoantigen ICA69, is also involved in DCV maturation and is recruited to Golgi membranes by activated RAB-2. Thus, we propose that RAB-2 and its effector RIC-19 are required for neuronal DCV maturation.
Figures





References
-
- Bermak J.C., Zhou Q.Y. 2001. Accessory proteins in the biogenesis of G protein-coupled receptors.Mol. Interv. 1:282–287 - PubMed
-
- Buffa L., Fuchs E., Pietropaolo M., Barr F., Solimena M. 2008. ICA69 is a novel Rab2 effector regulating ER-Golgi trafficking in insulinoma cells.Eur. J. Cell Biol. 87:197–209 doi:10.1016/j.ejcb.2007.11.003 - DOI - PubMed
-
- Burguete A.S., Fenn T.D., Brunger A.T., Pfeffer S.R. 2008. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185.Cell. 132:286–298 doi:10.1016/j.cell.2007.11.048 - DOI - PMC - PubMed
-
- Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. 1990. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments.Cell. 62:317–329 doi:10.1016/0092-8674(90)90369-P - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

