Genetic and functional interactions between the mitochondrial outer membrane proteins Tom6 and Sam37
- PMID: 19797086
- PMCID: PMC2772571
- DOI: 10.1128/MCB.00069-09
Genetic and functional interactions between the mitochondrial outer membrane proteins Tom6 and Sam37
Abstract
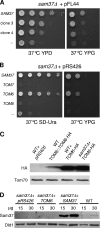
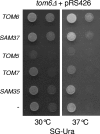
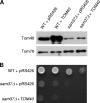
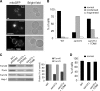
The TOM complex is the general mitochondrial entry site for newly synthesized proteins. Precursors of beta-barrel proteins initially follow this common pathway and are then relayed to the SAM/TOB complex, which mediates their integration into the outer membrane. Three proteins, Sam50 (Tob55), Sam35 (Tob38/Tom38), and Sam37 (Mas37), have been identified as the core constituents of the latter complex. Sam37 is essential for growth at elevated temperatures, but the function of the protein is currently unresolved. To identify interacting partners of Sam37 and thus shed light on its function, we screened for multicopy suppressors of sam37Delta. We identified the small subunit of the TOM complex, Tom6, as such a suppressor and found a tight genetic interaction between the two proteins. Overexpression of SAM37 suppresses the growth phenotype of tom6Delta, and cells lacking both genes are not viable. The ability of large amounts of Tom6 to suppress the sam37Delta phenotype can be linked to the capacity of Tom6 to stabilize Tom40, an essential beta-barrel protein which is the central component of the TOM complex. Our results suggest that Sam37 is required for growth at higher temperatures, since it enhances the biogenesis of Tom40, and this requirement can be overruled by improved stability of newly synthesized Tom40 molecules.
Figures







Similar articles
-
Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting β-barrel biogenesis.J Cell Biol. 2015 Sep 28;210(7):1047-54. doi: 10.1083/jcb.201504119. J Cell Biol. 2015. PMID: 26416958 Free PMC article.
-
The peripheral membrane subunits of the SAM complex function codependently in mitochondrial outer membrane biogenesis.Mol Biol Cell. 2008 Jan;19(1):126-36. doi: 10.1091/mbc.e07-08-0796. Epub 2007 Oct 31. Mol Biol Cell. 2008. PMID: 17978093 Free PMC article.
-
Alternative function for the mitochondrial SAM complex in biogenesis of alpha-helical TOM proteins.J Cell Biol. 2007 Dec 3;179(5):881-93. doi: 10.1083/jcb.200706043. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039934 Free PMC article.
-
Biogenesis of mitochondrial β-barrel membrane proteins.FEBS Open Bio. 2024 Oct;14(10):1595-1609. doi: 10.1002/2211-5463.13905. Epub 2024 Sep 29. FEBS Open Bio. 2024. PMID: 39343721 Free PMC article. Review.
-
How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane?J Cell Biol. 2005 Nov 7;171(3):419-23. doi: 10.1083/jcb.200507147. Epub 2005 Oct 31. J Cell Biol. 2005. PMID: 16260501 Free PMC article. Review.
Cited by
-
The Neurospora crassa TOB complex: analysis of the topology and function of Tob38 and Tob37.PLoS One. 2011;6(9):e25650. doi: 10.1371/journal.pone.0025650. Epub 2011 Sep 28. PLoS One. 2011. PMID: 21980517 Free PMC article.
-
Multispan mitochondrial outer membrane protein Ugo1 follows a unique Mim1-dependent import pathway.J Cell Biol. 2011 Aug 8;194(3):397-405. doi: 10.1083/jcb.201102041. J Cell Biol. 2011. PMID: 21825074 Free PMC article.
-
Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting β-barrel biogenesis.J Cell Biol. 2015 Sep 28;210(7):1047-54. doi: 10.1083/jcb.201504119. J Cell Biol. 2015. PMID: 26416958 Free PMC article.
-
Two-way communication between cell cycle and metabolism in budding yeast: what do we know?Front Microbiol. 2023 Jun 15;14:1187304. doi: 10.3389/fmicb.2023.1187304. eCollection 2023. Front Microbiol. 2023. PMID: 37396387 Free PMC article.
-
Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex.Genes (Basel). 2024 Nov 28;15(12):1534. doi: 10.3390/genes15121534. Genes (Basel). 2024. PMID: 39766801 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
