HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria
- PMID: 19797156
- PMCID: PMC2796736
- DOI: 10.1164/rccm.200907-1011OC
HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria
Abstract
Rationale: The risk of developing active tuberculosis in persons with latent Mycobacterium tuberculosis infection is substantially increased shortly after HIV-1 seroconversion. Immune responses in the lung are important to restrict the growth of M. tuberculosis to prevent the development of disease.
Objectives: To investigate innate and adaptive immune responses to M. tuberculosis in bronchoalveolar lavage from HIV-1-infected persons without active tuberculosis.
Methods: Peripheral blood was drawn and bronchoalveolar lavage (BAL) performed on healthy, HIV-1-uninfected (n = 21) and HIV-1-infected (n = 15) adults. Growth of M. tuberculosis was assessed in monocytes and alveolar macrophages. Cytokine expression by mycobacteria-specific CD4 and CD8 T cells was measured by intracellular cytokine staining or IFN-gamma ELISpot.
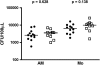
Measurements and main results: Mycobacterial growth in monocytes or alveolar macrophages from HIV-1-infected and -uninfected persons did not differ. Total CD4 T-cell frequencies in BAL were lower in HIV-1-infected than in HIV-1-uninfected persons (P < 0.001). Mycobacteria (bacillus Calmette-Guérin)-specific CD4 T-cell responses in BAL were severely impaired: Frequencies of cells expressing IFN-gamma or tumor necrosis factor (TNF)-alpha, as well as polyfunctional cells, expressing IFN-gamma, TNF-alpha, and IL-2 together, were lower in HIV-1-infected persons than in uninfected controls (P < 0.01 for all).
Conclusions: In addition to a total CD4 T-cell deficit, the function of mycobacteria-specific CD4 T cells is significantly impaired in the lung of HIV-1-infected persons, which may account for the HIV-1-associated elevated risk for developing tuberculosis.
Figures




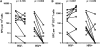

References
-
- Bates JH. Transmission and pathogenesis of tuberculosis. Clin Chest Med 1980;1:167–174. - PubMed
-
- Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol 2004;110:2–12. - PubMed
-
- Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol 1993;151:518–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

