Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs
- PMID: 19797161
- PMCID: PMC5451144
- DOI: 10.1164/rccm.200902-0215OC
Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs
Abstract
Rationale: Disordered extracellular matrix production is a feature of bronchopulmonary dysplasia (BPD). The basis of this phenomenon is not understood.
Objectives: To assess lysyl oxidase expression and activity in the injured developing lungs of newborn mice and of prematurely born infants with BPD or at risk for BPD.
Methods: Pulmonary lysyl oxidase and elastin gene and protein expression were assessed in newborn mice breathing 21 or 85% oxygen, in patients who died with BPD or were at risk for BPD, and in control patients. Signaling by transforming growth factor (TGF-beta) was preemptively blocked in mice exposed to hyperoxia using TGF-beta-neutralizing antibodies. Lysyl oxidase promoter activity was assessed using plasmids containing the lox or loxl1 promoters fused upstream of the firefly luciferase gene.
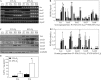
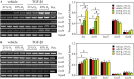
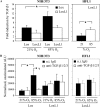
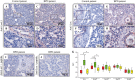
Measurements and main results: mRNA and protein levels and activity of lysyl oxidases (Lox, LoxL1, LoxL2) were elevated in the oxygen-injured lungs of newborn mice and infants with BPD or at risk for BPD. In oxygen-injured mouse lungs, increased TGF-beta signaling drove aberrant lox, but not loxl1 or loxl2, expression. Lox expression was also increased in oxygen-injured fibroblasts and pulmonary artery smooth muscle cells.
Conclusions: Lysyl oxidase expression and activity are dysregulated in BPD in injured developing mouse lungs and in prematurely born infants. In developing mouse lungs, aberrant TGF-beta signaling dysregulated lysyl oxidase expression. These data support the postulate that excessive stabilization of the extracellular matrix by excessive lysyl oxidase activity might impede the normal matrix remodeling that is required for pulmonary alveolarization and thereby contribute to the pathological pulmonary features of BPD.
Figures



References
-
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368. - PubMed
-
- Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. - PubMed
-
- Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate 2005;88:181–191. - PubMed
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. - PubMed
-
- Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005;67:623–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

