Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone
- PMID: 19797171
- PMCID: PMC2783580
- DOI: 10.1161/CIRCRESAHA.109.206318
Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone
Abstract
Rationale: Obesity is a risk factor for cardiovascular dysfunction, yet the underlying factors driving this impaired function remain poorly understood. Insulin resistance is a common pathology in obese patients and has been shown to impair vascular function. Whether insulin resistance or obesity, itself, is causal remains unclear.
Objective: The present study tested the hypothesis that insulin resistance is the underlying mediator for impaired NO-mediated dilation in obesity by genetic deletion of the insulin-desensitizing enzyme protein tyrosine phosphatase (PTP)1B in db/db mice.
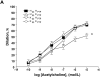
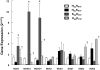
Methods and results: The db/db mouse is morbidly obese, insulin-resistant, and has tissue-specific elevation in PTP1B expression compared to lean controls. In db/db mice, PTP1B deletion improved glucose clearance, dyslipidemia, and insulin receptor signaling in muscle and fat. Hepatic insulin signaling in db/db mice was not improved by deletion of PTP1B, indicating specific amelioration of peripheral insulin resistance. Additionally, obese mice demonstrate an impaired endothelium dependent and independent vasodilation to acetylcholine and sodium nitroprusside, respectively. This impairment, which correlated with increased superoxide in the db/db mice, was corrected by superoxide scavenging. Increased superoxide production was associated with increased expression of NAD(P)H oxidase 1 and its molecular regulators, Noxo1 and Noxa1.
Conclusions: Deletion of PTP1B improved both endothelium dependent and independent NO-mediated dilation and reduced superoxide generation in db/db mice. PTP1B deletion did not affect any vascular function in lean mice. Taken together, these data reveal a role for peripheral insulin resistance as the mediator of vascular dysfunction in obesity.
Figures













Similar articles
-
NADPH oxidase 1 promotes hepatic steatosis in obese mice and is abrogated by augmented skeletal muscle mass.Am J Physiol Gastrointest Liver Physiol. 2024 Mar 1;326(3):G264-G273. doi: 10.1152/ajpgi.00153.2023. Epub 2024 Jan 23. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38258487 Free PMC article.
-
Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4.Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):777-84. doi: 10.1161/ATVBAHA.112.301087. Epub 2013 Feb 14. Arterioscler Thromb Vasc Biol. 2013. PMID: 23413427
-
Hepatic protein tyrosine phosphatase 1B (PTP1B) deficiency protects against obesity-induced endothelial dysfunction.Biochem Pharmacol. 2014 Dec 15;92(4):607-17. doi: 10.1016/j.bcp.2014.10.008. Epub 2014 Oct 30. Biochem Pharmacol. 2014. PMID: 25451690
-
[Role of protein tyrosine phosphatase 1B in the type 2 diabetes and obesity].Yi Chuan. 2004 Nov;26(6):941-6. Yi Chuan. 2004. PMID: 15640130 Review. Chinese.
-
Role of protein tyrosine phosphatase 1B in cardiovascular diseases.J Mol Cell Cardiol. 2016 Dec;101:50-57. doi: 10.1016/j.yjmcc.2016.09.002. Epub 2016 Sep 3. J Mol Cell Cardiol. 2016. PMID: 27596049 Review.
Cited by
-
Small extracellular vesicles from Ptpn1-deficient macrophages alleviate intestinal inflammation by reprogramming macrophage polarization via lactadherin enrichment.Redox Biol. 2022 Dec;58:102558. doi: 10.1016/j.redox.2022.102558. Epub 2022 Nov 28. Redox Biol. 2022. PMID: 36462232 Free PMC article.
-
Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation.Antioxidants (Basel). 2023 Mar 16;12(3):728. doi: 10.3390/antiox12030728. Antioxidants (Basel). 2023. PMID: 36978976 Free PMC article.
-
NADPH oxidase 1 promotes hepatic steatosis in obese mice and is abrogated by augmented skeletal muscle mass.Am J Physiol Gastrointest Liver Physiol. 2024 Mar 1;326(3):G264-G273. doi: 10.1152/ajpgi.00153.2023. Epub 2024 Jan 23. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38258487 Free PMC article.
-
Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress.Mol Cell Biochem. 2013 Jan;373(1-2):95-105. doi: 10.1007/s11010-012-1478-5. Epub 2012 Oct 17. Mol Cell Biochem. 2013. PMID: 23073711
-
Obesity alters the peripheral circadian clock in the aorta and microcirculation.Microcirculation. 2015 May;22(4):257-66. doi: 10.1111/micc.12192. Microcirculation. 2015. PMID: 25660131 Free PMC article.
References
-
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007 May;17(4):319–326. - PubMed
-
- Morisco C, Lembo G, Trimarco B. Insulin resistance and cardiovascular risk: New insights from molecular and cellular biology. Trends in cardiovascular medicine. 2006 Aug;16(6):183–188. - PubMed
-
- Goldstein BJ. Insulin resistance: from benign to type 2 diabetes mellitus. Rev Cardiovasc Med. 2003;4(Suppl 6):S3–10. - PubMed
-
- Verma S, Leung YM, Yao L, Battell M, Dumont AS, McNeill JH. Hyperinsulinemia superimposed on insulin resistance does not elevate blood pressure. Am J Hypertens. 2001;14(5 Pt 1):429–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

