The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion
- PMID: 19797241
- PMCID: PMC2763782
- DOI: 10.1152/ajpendo.00315.2009
The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion
Abstract
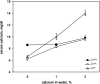
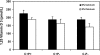
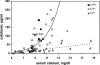
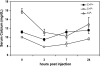
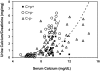
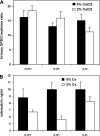
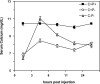
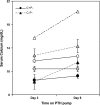
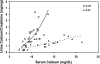
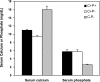
The calcium-sensing receptor (CaSR) controls parathyroid hormone (PTH) secretion, which, in turn, via direct and indirect actions on kidney, bone, and intestine, maintains a normal extracellular ionized calcium concentration (Ca(2+)(o)). There is less understanding of the CaSR's homeostatic importance outside of the parathyroid gland. We have employed single and double knockout mouse models, namely mice lacking PTH alone (CaSR(+/+) PTH(-/-), referred to as C(+)P(-)), lacking both CaSR and PTH (CaSR(-/-) PTH(-/-), C(-)P(-)) or wild-type (CaSR(+/+) PTH(+/+), C(+)P(+)) mice to study CaSR-specific functions without confounding CaSR-mediated changes in PTH. The mice received three hypercalcemic challenges: an oral Ca(2+) load, injection or constant infusion of PTH via osmotic pump, or a phosphate-deficient diet. C(-)P(-) mice show increased susceptibility to developing hypercalcemia with all three challenges compared with the other two genotypes, whereas C(+)P(-) mice defend against hypercalcemia similarly to C(+)P(+) mice. Reduced renal Ca(2+) clearance contributes to the intolerance of the C(-)P(-) mice to Ca(2+) loads, as they excrete less Ca(2+) at any given Ca(2+)(o) than the other two genotypes, confirming the CaSR's direct role in regulating renal Ca(2+) handling. In addition, C(+)P(+) and C(+)P(-), but not C(-)P(-), mice showed increases in serum calcitonin (CT) levels during hypercalcemia. The level of 1,25(OH)(2)D(3) in C(-)P(-) mice, in contrast, was similar to those in C(+)P(-) and C(+)P(+) mice during an oral Ca(2+) load, indicating that increased 1,25(OH)(2)D(3) production cannot account for the oral Ca(2+)-induced hypercalcemia in the C(-)P(-) mice. Thus, CaSR-stimulated PTH release serves as a "floor" to defend against hypocalcemia. In contrast, high-Ca(2+)(o)-induced inhibition of PTH is not required for a robust defense against hypercalcemia, at least in mice, whereas high-Ca(2+)(o)-stimulated, CaSR-mediated CT secretion and renal Ca(2+) excretion, and perhaps other factors, serve as a "ceiling" to limit hypercalcemia resulting from various types of hypercalcemic challenges.
Figures
References
-
- Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium 35: 229–237, 2004 - PubMed
-
- Breslau N, Moses AM, Weiner IM. The role of volume contraction in the hypocalciuric action of chlorothiazide. Kidney Int 10: 164–170, 1976 - PubMed
-
- Bringhurst FR, Demay MB, Kronenberg HM. Hormones, and disorders of mineral metabolism. In: Williams Textbook of Endocrinology (9th ed.), edited by Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Philadelphia, PA: WB Saunders, 1998, p. 1155–1209
-
- Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab 3: 122–133, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

