Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration
- PMID: 19797256
- PMCID: PMC2817575
- DOI: 10.1194/jlr.R002238
Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration
Abstract
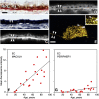
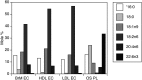
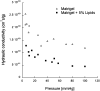
The largest risk factor for age-related macular degeneration (ARMD) is advanced age. With aging, there is a striking accumulation of neutral lipids in Bruch's membrane (BrM) of normal eye that continues through adulthood. This accumulation has the potential to significantly impact the physiology of the retinal pigment epithelium (RPE). It also ultimately leads to the creation of a lipid wall at the same locations where drusen and basal linear deposit, the pathognomonic extracellular, lipid-containing lesions of ARMD, subsequently form. Here, we summarize evidence obtained from light microscopy, ultrastructural studies, lipid histochemistry, assay of isolated lipoproteins, and gene expression analysis. These studies suggest that lipid deposition in BrM is at least partially due to accumulation of esterified cholesterol-rich, apolipoprotein B-containing lipoprotein particles produced by the RPE. Furthermore, we suggest that the formation of ARMD lesions and their aftermath may be a pathological response to the retention of a sub-endothelial apolipoprotein B lipoprotein, similar to a widely accepted model of atherosclerotic coronary artery disease (Tabas, I., K. J. Williams, and J. Borén. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116:1832-1844). This view provides a conceptual basis for the development of novel treatments that may benefit ARMD patients in the future.
Figures







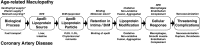
References
-
- Ethier C. R., Johnson M., Ruberti J. 2004. Ocular biomechanics and biotransport. Annu. Rev. Biomed. Eng. 6: 249–273. - PubMed
-
- Marshall J., Hussain A. A., Starita C., Moore D. J., Patmore A. L. 1998. Aging and Bruch's membrane. The Retinal Pigment Epithelium: Function and Disease. Marmor M. F., Wolfensberger T. J., Oxford University Press, New York: 669–692.
-
- Curcio C. A., Millican C. L., Bailey T., Kruth H. S. 2001. Accumulation of cholesterol with age in human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 42: 265–274. - PubMed
-
- Sivaprasad S., Bailey T. A., Chong V. N. 2005. Bruch's membrane and the vascular intima: is there a common basis for age-related changes and disease? Clin. Experiment. Ophthalmol. 33: 518–523. - PubMed
-
- Pauleikhoff D., Harper C. A., Marshall J., Bird A. C. 1990. Aging changes in Bruch's membrane: a histochemical and morphological study. Ophthalmology. 97: 171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

