A multicenter, randomized trial of treatment for mild gestational diabetes
- PMID: 19797280
- PMCID: PMC2804874
- DOI: 10.1056/NEJMoa0902430
A multicenter, randomized trial of treatment for mild gestational diabetes
Abstract
Background: It is uncertain whether treatment of mild gestational diabetes mellitus improves pregnancy outcomes.
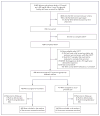
Methods: Women who were in the 24th to 31st week of gestation and who met the criteria for mild gestational diabetes mellitus (i.e., an abnormal result on an oral glucose-tolerance test but a fasting glucose level below 95 mg per deciliter [5.3 mmol per liter]) were randomly assigned to usual prenatal care (control group) or dietary intervention, self-monitoring of blood glucose, and insulin therapy, if necessary (treatment group). The primary outcome was a composite of stillbirth or perinatal death and neonatal complications, including hyperbilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma.
Results: A total of 958 women were randomly assigned to a study group--485 to the treatment group and 473 to the control group. We observed no significant difference between groups in the frequency of the composite outcome (32.4% and 37.0% in the treatment and control groups, respectively; P=0.14). There were no perinatal deaths. However, there were significant reductions with treatment as compared with usual care in several prespecified secondary outcomes, including mean birth weight (3302 vs. 3408 g), neonatal fat mass (427 vs. 464 g), the frequency of large-for-gestational-age infants (7.1% vs. 14.5%), birth weight greater than 4000 g (5.9% vs. 14.3%), shoulder dystocia (1.5% vs. 4.0%), and cesarean delivery (26.9% vs. 33.8%). Treatment of gestational diabetes mellitus, as compared with usual care, was also associated with reduced rates of preeclampsia and gestational hypertension (combined rates for the two conditions, 8.6% vs. 13.6%; P=0.01).
Conclusions: Although treatment of mild gestational diabetes mellitus did not significantly reduce the frequency of a composite outcome that included stillbirth or perinatal death and several neonatal complications, it did reduce the risks of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders. (ClinicalTrials.gov number, NCT00069576.)
2009 Massachusetts Medical Society
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Gestational diabetes--whom do we treat?N Engl J Med. 2009 Oct 1;361(14):1396-8. doi: 10.1056/NEJMe0907617. N Engl J Med. 2009. PMID: 19797287 No abstract available.
-
ACP Journal Club. Treatment of mild gestational diabetes did not prevent neonatal complications but reduced fetal overgrowth.Ann Intern Med. 2010 Jan 19;152(2):JC1-6. doi: 10.7326/0003-4819-152-2-201001190-02006. Ann Intern Med. 2010. PMID: 20083821 No abstract available.
-
Treatment for mild gestational diabetes.N Engl J Med. 2010 Jan 28;362(4):365-6; author reply 366-7. doi: 10.1056/NEJMc0910810. N Engl J Med. 2010. PMID: 20107225 No abstract available.
-
Treatment for mild gestational diabetes.N Engl J Med. 2010 Jan 28;362(4):366; author reply 366-7. N Engl J Med. 2010. PMID: 20112447 No abstract available.
-
Gestational diabetes mellitus: can we reach consensus?Curr Diab Rep. 2010 Aug;10(4):252-4. doi: 10.1007/s11892-010-0117-3. Curr Diab Rep. 2010. PMID: 20443085 No abstract available.
References
-
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–S48. - PubMed
-
- Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–8. - PubMed
-
- O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–85. - PubMed
-
- Buchanan TA, Kjos SL. Gestational diabetes: risk or myth? J Clin Endocrinol Metab. 1999;84:1854–7. - PubMed
-
- Langer O, Yogev Y, Most O, Yexakis EMJ. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192:989–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- UL1-RR024989/RR/NCRR NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD053118/HD/NICHD NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- M01-RR00034/RR/NCRR NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- M01 RR000034/RR/NCRR NIH HHS/United States
- UL1-RR025764/RR/NCRR NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD53118/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- C06 RR011234/RR/NCRR NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- M01-RR00080/RR/NCRR NIH HHS/United States
- R24 HD050924/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- HD53097/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous