Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability
- PMID: 19797520
- PMCID: PMC2786294
- DOI: 10.1182/blood-2009-06-228940
Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability
Abstract
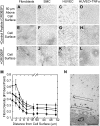
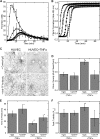
Fibrin is essential for hemostasis; however, abnormal fibrin formation is hypothesized to increase thrombotic risk. We previously showed that in situ thrombin generation on a cell's surface modulates the 3-dimensional structure and stability of the fibrin network. Currently, we compared the abilities of extravascular and intravascular cells to support fibrin formation, structure, and stability. Extravascular cells (fibroblasts, smooth muscle) supported formation of dense fibrin networks that resisted fibrinolysis, whereas unstimulated intravascular (endothelial) cells produced coarse networks that were susceptible to fibrinolysis. All 3 cell types produced a fibrin structural gradient, with a denser network near, versus distal to, the cell surface. Although fibrin structure depended on cellular procoagulant activity, it did not reflect interactions between integrins and fibrin. These findings contrasted with those on platelets, which influenced fibrin structure via interactions between beta3 integrins and fibrin. Inflammatory cytokines that induced prothrombotic activity on endothelial cells caused the production of abnormally dense fibrin networks that resisted fibrinolysis. Blocking tissue factor activity significantly reduced the density and stability of fibrin networks produced by cytokine-stimulated endothelial cells. Together, these findings indicate fibrin structure and stability reflect the procoagulant phenotype of the endogenous cells, and suggest abnormal fibrin structure is a novel link between inflammation and thrombosis.
Figures





References
-
- Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105(3):1102–1105. - PubMed
-
- Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26(11):2567–2573. - PubMed
-
- Blombäck B, Carlsson K, Hessel B, Liljeborg A, Procyk R, Aslund N. Native fibrin gel networks observed by 3D microscopy, permeation and turbidity. Biochim Biophys Acta. 1989;997(1-2):96–110. - PubMed
-
- Wolberg AS, Allen GA, Monroe DM, Hedner U, Roberts HR, Hoffman M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br J Haematol. 2005;131(5):645–655. - PubMed
-
- Collet JP, Park D, Lesty C, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20(5):1354–1361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

