Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection
- PMID: 19797524
- PMCID: PMC2786293
- DOI: 10.1182/blood-2009-05-220806
Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection
Abstract
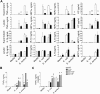
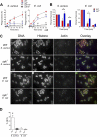
Leukocyte-specific CD18 integrins are critical in mediating cell recruitment and activation during host defense responses to bacterial infection. The signaling pathways downstream of CD18 integrins are dependent on the spleen tyrosine kinase, Syk. To investigate the role integrin signaling plays in host defense, we examined the responses of Syk-deficient neutrophils to bacterial challenge with serum-opsonized Staphylococcus aureus and Escherichia coli. Syk-conditional knockout mice lacking this kinase specifically in myeloid cells or just neutrophils were also used to investigate host responses in vivo. Syk-deficient neutrophils manifested impaired exocytosis of secondary and tertiary granules, reduced cytokine release, and very poor activation of the NADPH oxidase in response to serum-opsonized S aureus and E coli. These functional defects correlated with impaired activation of c-Cbl, Pyk2, Erk1/2, and p38 kinases. Bacterial phagocytosis, neutrophil extracellular trap formation, and killing were also reduced in Syk-deficient cells, with a more profound effect after S aureus challenge. In vivo, loss of Syk in myeloid cells or specifically in neutrophils resulted in reduced clearance of S aureus after subcutaneous or intraperitoneal infection, despite normal recruitment of inflammatory cells. These results indicate that loss of Syk kinase-mediated integrin signaling impairs leukocyte activation, leading to reduced host defense responses.
Figures





References
-
- Etzioni A, Doerschuk CM, Harlan JM. Of man and mouse: leukocyte and endothelial adhesion molecule deficiencies. Blood. 1999;94(10):3281–3288. - PubMed
-
- Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9(1):30–35. - PubMed
-
- Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113(4):620–626. - PubMed
-
- Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11(9):621–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

