Identification of a myeloid committed progenitor as the cancer-initiating cell in acute promyelocytic leukemia
- PMID: 19797526
- PMCID: PMC2798860
- DOI: 10.1182/blood-2008-10-182071
Identification of a myeloid committed progenitor as the cancer-initiating cell in acute promyelocytic leukemia
Abstract
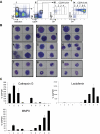
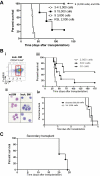
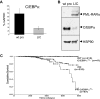
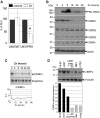
Acute promyelocytic leukemia (APL) is characterized by a block in differentiation and accumulation of promyelocytes in the bone marrow and blood. The majority of APL patients harbor the t(15:17) translocation leading to expression of the fusion protein promyelocytic-retinoic acid receptor alpha. Treatment with retinoic acid leads to degradation of promyelocytic-retinoic acid receptor alpha protein and disappearance of leukemic cells; however, 30% of APL patients relapse after treatment. One potential mechanism for relapse is the persistence of cancer "stem" cells in hematopoietic organs after treatment. Using a novel sorting strategy we developed to isolate murine myeloid cells at distinct stages of differentiation, we identified a population of committed myeloid cells (CD34(+), c-kit(+), FcgammaRIII/II(+), Gr1(int)) that accumulates in the spleen and bone marrow in a murine model of APL. We observed that these cells are capable of efficiently generating leukemia in recipient mice, demonstrating that this population represents the APL cancer-initiating cell. These cells down-regulate the transcription factor CCAAT/enhancer binding protein alpha (C/EBPalpha) possibly through a methylation-dependent mechanism, indicating that C/EBPalpha deregulation contributes to transformation of APL cancer-initiating cells. Our findings provide further understanding of the biology of APL by demonstrating that a committed transformed progenitor can initiate and propagate the disease.
Figures

Similar articles
-
BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARalpha) to block neutrophil differentiation and initiate acute leukemia.J Exp Med. 2001 Feb 19;193(4):531-43. doi: 10.1084/jem.193.4.531. J Exp Med. 2001. PMID: 11181704 Free PMC article.
-
PML-RARalpha initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors.Leukemia. 2009 Aug;23(8):1462-71. doi: 10.1038/leu.2009.63. Epub 2009 Mar 26. Leukemia. 2009. PMID: 19322209 Free PMC article.
-
DNA methylation changes are a late event in acute promyelocytic leukemia and coincide with loss of transcription factor binding.Blood. 2013 Jan 3;121(1):178-87. doi: 10.1182/blood-2012-08-448860. Epub 2012 Nov 14. Blood. 2013. PMID: 23152544
-
The impact of molecularly targeted therapies upon the understanding of leukemogenesis and the role of hematopoietic stem cell transplantation in acute promyelocytic leukemia.Curr Stem Cell Res Ther. 2010 Dec;5(4):372-8. doi: 10.2174/157488810793351695. Curr Stem Cell Res Ther. 2010. PMID: 20528759 Review.
-
Understanding the molecular pathogenesis of acute promyelocytic leukemia.Best Pract Res Clin Haematol. 2014 Mar;27(1):3-9. doi: 10.1016/j.beha.2014.04.006. Epub 2014 Apr 13. Best Pract Res Clin Haematol. 2014. PMID: 24907012 Review.
Cited by
-
Acute promyelocytic leukaemia: novel insights into the mechanisms of cure.Nat Rev Cancer. 2010 Nov;10(11):775-83. doi: 10.1038/nrc2943. Epub 2010 Oct 22. Nat Rev Cancer. 2010. PMID: 20966922 Review.
-
C/EBPα deregulation as a paradigm for leukemogenesis.Leukemia. 2017 Nov;31(11):2279-2285. doi: 10.1038/leu.2017.229. Epub 2017 Jul 19. Leukemia. 2017. PMID: 28720765 Free PMC article. Review.
-
Oroxylin A, a natural anticancer flavonoid compound, induces differentiation of t(8;21)-positive Kasumi-1 and primary acute myeloid leukemia cells.J Cancer Res Clin Oncol. 2016 Jul;142(7):1449-59. doi: 10.1007/s00432-016-2160-1. Epub 2016 Apr 16. J Cancer Res Clin Oncol. 2016. PMID: 27085528 Free PMC article.
-
Tumor cells positive and negative for the common cancer stem cell markers are capable of initiating tumor growth and generating both progenies.PLoS One. 2013;8(1):e54579. doi: 10.1371/journal.pone.0054579. Epub 2013 Jan 21. PLoS One. 2013. PMID: 23349932 Free PMC article.
-
Caloric restriction leads to druggable LSD1-dependent cancer stem cells expansion.Nat Commun. 2024 Jan 27;15(1):828. doi: 10.1038/s41467-023-44348-y. Nat Commun. 2024. PMID: 38280853 Free PMC article.
References
-
- de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347(6293):558–561. - PubMed
-
- Grimwade D, Biondi A, Mozziconacci MJ, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Francais de Cytogenetique Hematologique, Groupe de Francais d'Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action Molecular Cytogenetic Diagnosis in Haematological Malignancies. Blood. 2000;96(4):1297–1308. - PubMed
-
- Grisolano JL, Wesselschmidt RL, Pelicci PG, Ley TJ. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RAR alpha under control of cathepsin G regulatory sequences. Blood. 1997;89(2):376–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

