Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy
- PMID: 19797619
- PMCID: PMC2802480
- DOI: 10.1124/jpet.109.160382
Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy
Abstract
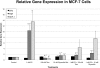
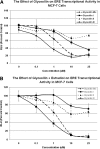
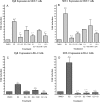
Glyceollins, a group of novel phytoalexins isolated from activated soy, have recently been demonstrated to be novel antiestrogens that bind to the estrogen receptor (ER) and inhibit estrogen-induced tumor progression. Our previous publications have focused specifically on inhibition of tumor formation and growth by the glyceollin mixture, which contains three glyceollin isomers (I, II, and III). Here, we show the glyceollin mixture is also effective as a potential antiestrogenic, therapeutic agent that prevents estrogen-stimulated tumorigenesis and displays a differential pattern of gene expression from tamoxifen. By isolating the individual glyceollin isomers (I, II, and III), we have identified the active antiestrogenic component by using competition binding assays with human ERalpha and in an estrogen-responsive element-based luciferase reporter assay. We identified glyceollin I as the active component of the combined glyceollin mixture. Ligand-receptor modeling (docking) of glyceollin I, II, and III within the ERalpha ligand binding cavity demonstrates a unique type II antiestrogenic confirmation adopted by glyceollin I but not isomers II and III. We further compared the effects of glyceollin I to the antiestrogens, 4-hydroxytamoxifen and ICI 182,780 (fulvestrant), in MCF-7 breast cancer cells and BG-1 ovarian cancer cells on 17beta-estradiol-stimulated expression of progesterone receptor and stromal derived factor-1alpha. Our results establish a novel inhibition of ER-mediated gene expression and cell proliferation/survival. Glyceollin I may represent an important component of a phytoalexin-enriched food (activated) diet in terms of chemoprevention as well as a novel therapeutic agent for hormone-dependent tumors.
Figures



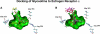

References
-
- Ali S, Coombes RC. (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112 - PubMed
-
- Banks SW, Dewick PM. (1983) Biosynthesis of glyceollins I, II and III in soybean. Phytochemistry 22:2729–2733
-
- Barnes S. (1998) Phytoestrogens and breast cancer. Baillieres Clin Endocrinol Metab 12:559–579 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

