Noninvasive real-time measurement of nasal mucociliary clearance in mice by pinhole gamma scintigraphy
- PMID: 19797687
- PMCID: PMC2885071
- DOI: 10.1152/japplphysiol.00669.2009
Noninvasive real-time measurement of nasal mucociliary clearance in mice by pinhole gamma scintigraphy
Abstract
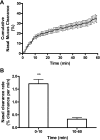
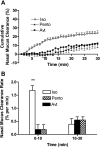
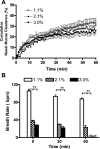
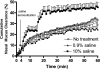
Mucociliary clearance (MCC) is the key defense mechanism in the upper airways, as the removal of debris-laden mucus in the sinuses completely depends on MCC. So far, how the nasal MCC is regulated remains unknown. Recently, mice deficient in genes encoding the components of MCC apparatus have been generated, which will allow investigators to conduct more in-depth nasal MCC studies. However, the methodology necessary to comprehensively evaluate the nasal MCC in this species is not well established. We therefore developed a novel method to measure nasal MCC in live mice using pinhole gamma camera. Insoluble radiolabeled particles were delivered into the noses of lightly anesthetized mice. The nasal clearance of these particles was measured continuously in a real-time manner. The effect of three different anesthetics-avertin, pentobarbital, and isoflurane-on nasal MCC was also determined. In mice anesthetized by 1.1% isoflurane, radiolabeled particles were immediately moved into the oropharynx, which was significantly accelerated by the treatment of hypertonic but not isotonic saline. According to the clearance rate, the mouse nasal MCC presented two distinct phases: a rapid phase and a slow phase. In addition, we found that isoflurane had a very small inhibitory effect on nasal MCC vs. both avertin and pentobarbital. This was further supported by its dose response. Collectively, we have developed a noninvasive method to monitor the real-time nasal MCC in live mice under physiological conditions. It provides more comprehensive evaluation on nasal MCC rather than assessing a single component of the MCC apparatus in isolation.
Figures


Comment in
-
A better picture of clearance in the nose.J Appl Physiol (1985). 2010 Jan;108(1):1-2. doi: 10.1152/japplphysiol.01284.2009. Epub 2009 Nov 19. J Appl Physiol (1985). 2010. PMID: 19926823 No abstract available.
Similar articles
-
A better picture of clearance in the nose.J Appl Physiol (1985). 2010 Jan;108(1):1-2. doi: 10.1152/japplphysiol.01284.2009. Epub 2009 Nov 19. J Appl Physiol (1985). 2010. PMID: 19926823 No abstract available.
-
Mucociliary Clearance in Mice Measured by Tracking Trans-tracheal Fluorescence of Nasally Aerosolized Beads.Sci Rep. 2018 Oct 3;8(1):14744. doi: 10.1038/s41598-018-33053-2. Sci Rep. 2018. PMID: 30282981 Free PMC article.
-
BPIFB1 loss alters airway mucus properties and diminishes mucociliary clearance.Am J Physiol Lung Cell Mol Physiol. 2023 Dec 1;325(6):L765-L775. doi: 10.1152/ajplung.00390.2022. Epub 2023 Oct 17. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37847709 Free PMC article.
-
Mucociliary clearance in cystic fibrosis.Pediatr Pulmonol. 2002 Apr;33(4):293-306. doi: 10.1002/ppul.10079. Pediatr Pulmonol. 2002. PMID: 11921459 Review.
-
Effects of drugs on mucus clearance.Eur Respir J. 1999 Aug;14(2):452-67. doi: 10.1034/j.1399-3003.1999.14b35.x. Eur Respir J. 1999. PMID: 10515429 Review.
Cited by
-
Method for Measuring Mucociliary Clearance and Cilia-generated Flow in Mice by ex vivo Imaging.Bio Protoc. 2020 Mar 20;10(6):e3554. doi: 10.21769/BioProtoc.3554. eCollection 2020 Mar 20. Bio Protoc. 2020. PMID: 33659527 Free PMC article.
-
PCD Genes-From Patients to Model Organisms and Back to Humans.Int J Mol Sci. 2022 Feb 3;23(3):1749. doi: 10.3390/ijms23031749. Int J Mol Sci. 2022. PMID: 35163666 Free PMC article. Review.
-
Avertin®, but Not Volatile Anesthetics Addressing the Two-Pore Domain K+ Channel, TASK-1, Slows Down Cilia-Driven Particle Transport in the Mouse Trachea.PLoS One. 2016 Dec 8;11(12):e0167919. doi: 10.1371/journal.pone.0167919. eCollection 2016. PLoS One. 2016. PMID: 27930725 Free PMC article.
-
Particle coating alters mucociliary transit in excised rat trachea: A synchrotron X-ray imaging study.Sci Rep. 2019 Jul 29;9(1):10983. doi: 10.1038/s41598-019-47465-1. Sci Rep. 2019. PMID: 31358851 Free PMC article.
-
Non-invasive airway health assessment: synchrotron imaging reveals effects of rehydrating treatments on mucociliary transit in-vivo.Sci Rep. 2014 Jan 14;4:3689. doi: 10.1038/srep03689. Sci Rep. 2014. PMID: 24418935 Free PMC article.
References
-
- Antunes MB, Cohen NA. Mucociliary clearance—a critical upper airway host defense mechanism and methods of assessment. Current Opin Allergy Clin Immunol 7: 5–10, 2007 - PubMed
-
- Belvisi MG. Overview of the innervation of the lung. Current Opin Pharmacol 2: 211–215, 2002 - PubMed
-
- Braverman I, Wright ED, Wang CG, Eidelman D, Frenkiel S. Human nasal ciliary-beat frequency in normal and chronic sinusitis subjects. J Otolaryngol 27: 145–152, 1998 - PubMed
-
- Bridger GP, Proctor DF. Mucociliary function in the dog's larynx and trachea. Laryngoscope 82: 218–224, 1972 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

