Dendritic cells as targets for therapy in rheumatoid arthritis
- PMID: 19798032
- PMCID: PMC2884969
- DOI: 10.1038/nrrheum.2009.185
Dendritic cells as targets for therapy in rheumatoid arthritis
Abstract
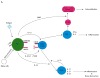
Dendritic cells (DCs) are central in inducing immunity and in mediating immune tolerance in their role as professional antigen-presenting cells. In the absence of DCs, a fatal autoimmunity develops in animal models. Although the role of DCs has been investigated extensively in the pathogenesis of rheumatoid arthritis (RA), it remains unclear whether DCs initiate autoimmunity in this disease. Nevertheless, evidence points towards a significant role for DCs in disease maintenance and progression. Current biologic therapies target cytokine products of antigen-presenting cells, such as tumor necrosis factor, interleukin-1 and interleukin-6. Emerging therapies for RA exploit the tolerogenic capacity of DCs. 'Tolerogenic' DCs can be generated from myeloid precursors ex vivo, loaded with antigen, and manipulated to suppress autoimmune responses in vivo, through the induction of activation-induced cell death, anergy, and/or regulatory T cells. Cells that are primed by DCs, such as B cells, type 1 and type 17 T helper cells, and that have been implicated in certain models of autoimmunity, are also being considered as additional targets for immune-based therapy. Studies to validate these approaches to ameliorate autoimmunity will be necessary before their application in the clinic.
Conflict of interest statement
Figures

Similar articles
-
Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy.Arthritis Rheum. 2010 Jan;62(1):53-63. doi: 10.1002/art.25037. Arthritis Rheum. 2010. PMID: 20039433
-
Tolerogenic dendritic cells and rheumatoid arthritis: current status and perspectives.Rheumatol Int. 2012 Apr;32(4):837-44. doi: 10.1007/s00296-011-2133-2. Epub 2011 Sep 9. Rheumatol Int. 2012. PMID: 21904923 Review.
-
Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells.Blood. 2006 May 1;107(9):3632-8. doi: 10.1182/blood-2005-11-4497. Epub 2006 Jan 5. Blood. 2006. PMID: 16397128 Free PMC article.
-
Targeting DCs for Tolerance Induction: Don't Lose Sight of the Neutrophils.Front Immunol. 2021 Oct 5;12:732992. doi: 10.3389/fimmu.2021.732992. eCollection 2021. Front Immunol. 2021. PMID: 34675923 Free PMC article. Review.
-
Regulatory dendritic cells in autoimmunity: A comprehensive review.J Autoimmun. 2015 Sep;63:1-12. doi: 10.1016/j.jaut.2015.07.011. Epub 2015 Aug 5. J Autoimmun. 2015. PMID: 26255250 Review.
Cited by
-
Current Immunotherapy Strategies for Rheumatoid Arthritis: The Immunoengineering and Delivery Systems.Research (Wash D C). 2023 Oct 17;6:0220. doi: 10.34133/research.0220. eCollection 2023. Research (Wash D C). 2023. PMID: 39902178 Free PMC article. Review.
-
Biomaterials for Modulating the Immune Microenvironment in Rheumatoid Arthritis.BME Front. 2025 Mar 10;6:0102. doi: 10.34133/bmef.0102. eCollection 2025. BME Front. 2025. PMID: 40065832 Free PMC article. Review.
-
Innate immunity drives pathogenesis of rheumatoid arthritis.Biomed J. 2021 Apr;44(2):172-182. doi: 10.1016/j.bj.2020.06.010. Epub 2020 Jul 8. Biomed J. 2021. PMID: 32798211 Free PMC article. Review.
-
Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):899-904. doi: 10.1073/pnas.1118462109. Epub 2012 Jan 4. Proc Natl Acad Sci U S A. 2012. PMID: 22219356 Free PMC article.
-
CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis.J Pers Med. 2022 Nov 9;12(11):1875. doi: 10.3390/jpm12111875. J Pers Med. 2022. PMID: 36579595 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

