The structure of an integrin/talin complex reveals the basis of inside-out signal transduction
- PMID: 19798053
- PMCID: PMC2782098
- DOI: 10.1038/emboj.2009.287
The structure of an integrin/talin complex reveals the basis of inside-out signal transduction
Abstract
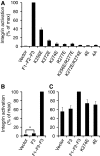
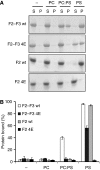
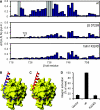
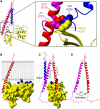
Fundamental to cell adhesion and migration, integrins are large heterodimeric membrane proteins that uniquely mediate inside-out signal transduction, whereby adhesion to the extracellular matrix is activated from within the cell by direct binding of talin to the cytoplasmic tail of the beta integrin subunit. Here, we report the first structure of talin bound to an authentic full-length beta integrin tail. Using biophysical and whole cell measurements, we show that a specific ionic interaction between the talin F3 domain and the membrane-proximal helix of the beta tail disrupts an integrin alpha/beta salt bridge that helps maintain the integrin inactive state. Second, we identify a positively charged surface on the talin F2 domain that precisely orients talin to disrupt the heterodimeric integrin transmembrane (TM) complex. These results show key structural features that explain the ability of talin to mediate inside-out TM signalling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Abramoff MD, Magelhaes PJ, Ram SJ (1994) Image processing with ImageJ. Biophoton Int 11: 36–42
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58(Part 11): 1948–1954 - PubMed
-
- Ayed A, Mulder FA, Yi GS, Lu Y, Kay LE, Arrowsmith CH (2001) Latent and active p53 are identical in conformation. Nat Struct Biol 8: 756–760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

