Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes
- PMID: 19798428
- PMCID: PMC2745659
- DOI: 10.1371/journal.ppat.1000604
Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes
Abstract
The enormous toll on human life during the 1918-1919 Spanish influenza pandemic is a constant reminder of the potential lethality of influenza viruses. With the declaration by the World Health Organization of a new H1N1 influenza virus pandemic, and with continued human cases of highly pathogenic H5N1 avian influenza virus infection, a better understanding of the host response to highly pathogenic influenza viruses is essential. To this end, we compared pathology and global gene expression profiles in bronchial tissue from macaques infected with either the reconstructed 1918 pandemic virus or the highly pathogenic avian H5N1 virus A/Vietnam/1203/04. Severe pathology was observed in respiratory tissues from 1918 virus-infected animals as early as 12 hours after infection, and pathology steadily increased at later time points. Although tissues from animals infected with A/Vietnam/1203/04 also showed clear signs of pathology early on, less pathology was observed at later time points, and there was evidence of tissue repair. Global transcriptional profiles revealed that specific groups of genes associated with inflammation and cell death were up-regulated in bronchial tissues from animals infected with the 1918 virus but down-regulated in animals infected with A/Vietnam/1203/04. Importantly, the 1918 virus up-regulated key components of the inflammasome, NLRP3 and IL-1beta, whereas these genes were down-regulated by A/Vietnam/1203/04 early after infection. TUNEL assays revealed that both viruses elicited an apoptotic response in lungs and bronchi, although the response occurred earlier during 1918 virus infection. Our findings suggest that the severity of disease in 1918 virus-infected macaques is a consequence of the early up-regulation of cell death and inflammatory related genes, in which additive or synergistic effects likely dictate the severity of tissue damage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



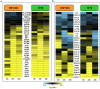
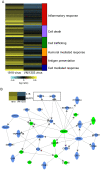

References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. - PubMed
-
- Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. - PubMed
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med. 2009;360:2605–2615. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

