Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation
- PMID: 19798429
- PMCID: PMC2745661
- DOI: 10.1371/journal.ppat.1000605
Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation
Abstract
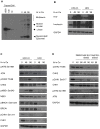
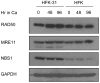
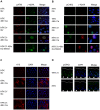
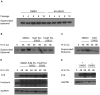
Human papillomaviruses (HPV) are the causative agents of cervical cancers. The infectious HPV life cycle is closely linked to the differentiation state of the host epithelia, with viral genome amplification, late gene expression and virion production restricted to suprabasal cells. The E6 and E7 proteins provide an environment conducive to DNA synthesis upon differentiation, but little is known concerning the mechanisms that regulate productive viral genome amplification. Using keratinocytes that stably maintain HPV-31 episomes, and chemical inhibitors, we demonstrate that viral proteins activate the ATM DNA damage response in differentiating cells, as indicated by phosphorylation of CHK2, BRCA1 and NBS1. This activation is necessary for viral genome amplification, as well as for formation of viral replication foci. In contrast, inhibition of ATM kinase activity in undifferentiated keratinocytes had no effect on the stable maintenance of viral genomes. Previous studies have shown that HPVs induce low levels of caspase 3/7 activation upon differentiation and that this is important for cleavage of the E1 replication protein and genome amplification. Our studies demonstrate that caspase cleavage is induced upon differentiation of HPV positive cells through the action of the DNA damage protein kinase CHK2, which may be activated as a result of E7 binding to the ATM kinase. These findings identify a major regulatory mechanism responsible for productive HPV replication in differentiating cells. Our results have potential implications for the development of anti-viral therapies to treat HPV infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Human papillomaviruses activate and recruit SMC1 cohesin proteins for the differentiation-dependent life cycle through association with CTCF insulators.PLoS Pathog. 2015 Apr 13;11(4):e1004763. doi: 10.1371/journal.ppat.1004763. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25875106 Free PMC article.
-
STAT-5 Regulates Transcription of the Topoisomerase IIβ-Binding Protein 1 (TopBP1) Gene To Activate the ATR Pathway and Promote Human Papillomavirus Replication.mBio. 2015 Dec 22;6(6):e02006-15. doi: 10.1128/mBio.02006-15. mBio. 2015. PMID: 26695634 Free PMC article.
-
FANCD2 Binds Human Papillomavirus Genomes and Associates with a Distinct Set of DNA Repair Proteins to Regulate Viral Replication.mBio. 2017 Feb 14;8(1):e02340-16. doi: 10.1128/mBio.02340-16. mBio. 2017. PMID: 28196964 Free PMC article.
-
Modulation of the DNA damage response during the life cycle of human papillomaviruses.Virus Res. 2017 Mar 2;231:41-49. doi: 10.1016/j.virusres.2016.11.006. Epub 2016 Nov 9. Virus Res. 2017. PMID: 27836727 Free PMC article. Review.
-
DNA damage response is hijacked by human papillomaviruses to complete their life cycle.J Zhejiang Univ Sci B. 2017 Mar;18(3):215-232. doi: 10.1631/jzus.B1600306. J Zhejiang Univ Sci B. 2017. PMID: 28271657 Free PMC article. Review.
Cited by
-
DNA Damage Reduces the Quality, but Not the Quantity of Human Papillomavirus 16 E1 and E2 DNA Replication.Viruses. 2016 Jun 22;8(6):175. doi: 10.3390/v8060175. Viruses. 2016. PMID: 27338449 Free PMC article.
-
Activation of DNA damage repair factors in HPV positive oropharyngeal cancers.Virology. 2020 Aug;547:27-34. doi: 10.1016/j.virol.2020.05.003. Epub 2020 May 22. Virology. 2020. PMID: 32560902 Free PMC article.
-
Human papillomavirus episome stability is reduced by aphidicolin and controlled by DNA damage response pathways.J Virol. 2013 Apr;87(7):3979-89. doi: 10.1128/JVI.03473-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365423 Free PMC article.
-
Human Papillomaviruses-Related Cancers: An Update on the Presence and Prevention Strategies in the Middle East and North African Regions.Pathogens. 2022 Nov 19;11(11):1380. doi: 10.3390/pathogens11111380. Pathogens. 2022. PMID: 36422631 Free PMC article. Review.
-
Vorinostat, a pan-HDAC inhibitor, abrogates productive HPV-18 DNA amplification.Proc Natl Acad Sci U S A. 2018 Nov 20;115(47):E11138-E11147. doi: 10.1073/pnas.1801156115. Epub 2018 Nov 1. Proc Natl Acad Sci U S A. 2018. PMID: 30385631 Free PMC article.
References
-
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Howely PM. Papillomaviridae: the viruses and their replication. 1996
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

